Abstract
Two methods were used to compare the biodegradation of six polychlorinated biphenyl (PCB) congeners by 12 white rot fungi. Four fungi were found to be more active than Phanerochaete chrysosporium ATCC 24725. Biodegradation of the following congeners was monitored by gas chromatography: 2,3-dichlorobiphenyl, 4,4′-dichlorobiphenyl, 2,4′,5-trichlorobiphenyl (2,4′,5-TCB), 2,2′,4,4′-tetrachlorobiphenyl, 2,2′,5,5′-tetrachlorobiphenyl, and 2,2′,4,4′,5,5′-hexachlorobiphenyl. The congener tested for mineralization was 2,4′,5-[U-14C]TCB. Culture supernatants were also assayed for lignin peroxidase and manganese peroxidase activities. Of the fungi tested, two strains of Bjerkandera adusta (UAMH 8258 and UAMH 7308), one strain of Pleurotus ostreatus (UAMH 7964), and Trametes versicolor UAMH 8272 gave the highest biodegradation and mineralization. P. chrysosporium ATCC 24725, a strain frequently used in studies of PCB degradation, gave the lowest mineralization and biodegradation activities of the 12 fungi reported here. Low but detectable levels of lignin peroxidase and manganese peroxidase activity were present in culture supernatants, but no correlation was observed among any combination of PCB congener biodegradation, mineralization, and lignin peroxidase or manganese peroxidase activity. With the exception of P. chrysosporium, congener loss ranged from 40 to 96%; however, these values varied due to nonspecific congener binding to fungal biomass and glassware. Mineralization was much lower, ≤11%, because it measures a complete oxidation of at least part of the congener molecule but the results were more consistent and therefore more reliable in assessment of PCB biodegradation.
Polychlorinated biphenyls (PCBs) are produced by chlorination of biphenyl, resulting in up to 209 different congeners. Commercial mixtures range from light oily fluids to waxes, and their physical properties make them useful as heat transfer fluids, hydraulic fluids, solvent extenders, plasticizers, flame retardants, organic diluents, and dielectric fluids (1, 21). Approximately 24 million lb are in the North American environment (19). The stability and hydrophobic nature of these compounds make them a persistent environmental hazard.
To date, bacterial transformations have been the main focus of PCB degradation research. Aerobic bacteria use a biphenyl-induced dioxygenase enzyme system to attack less-chlorinated congeners (mono- to hexachlorobiphenyls) (1, 5, 7, 8, 22). Although more-chlorinated congeners are recalcitrant to aerobic bacterial degradation, microorganisms in anaerobic river sediments reductively dechlorinate these compounds, mainly removing the meta and para chlorines (1, 6, 10, 33, 34).
The degradation of PCBs by white rot fungi has been known since 1985 (11, 18). Many fungi have been tested for their ability to degrade PCBs, including the white rot fungi Coriolus versicolor (18), Coriolopsis polysona (41), Funalia gallica (18), Hirneola nigricans (35), Lentinus edodes (35), Phanerochaete chrysosporium (3, 11, 14, 17, 18, 35, 39, 41–43), Phlebia brevispora (18), Pleurotus ostreatus (35, 43), Poria cinerescens (18), Px strain (possibly Lentinus tigrinus) (35), and Trametes versicolor (41, 43). There have also been studies of PCB metabolism by ectomycorrhizal fungi (17) and other fungi such as Aspergillus flavus (32), Aspergillus niger (15), Aureobasidium pullulans (18), Candida boidinii (35), Candida lipolytica (35), Cunninghamella elegans (16), and Saccharomyces cerevisiae (18, 38). The mechanism of PCB biodegradation has not been definitively determined for any fungi. White rot fungi produce several nonspecific extracellular enzymes which have been the subject of extensive research. These nonspecific peroxidases are normally involved in lignin degradation but can oxidize a wide range of aromatic compounds including polycyclic aromatic hydrocarbons (37). Two peroxidases, lignin peroxidase (LiP) and Mn peroxidase (MnP), are secreted into the environment of the fungus under conditions of nitrogen limitation in P. chrysosporium (23, 25, 27, 29) but are not stress related in fungi such as Bjerkandera adusta or T. versicolor (12, 30).
Two approaches have been used to determine the biodegradability of PCBs by fungi: (i) loss of the parent congener analyzed by gas chromatography (GC) (17, 32, 35, 42, 43) and (ii) mineralization experiments in which the 14C of the universally labeled 14C parent congener is recovered as 14CO2 (11, 14, 18, 39, 41). In the first method, the loss of a peak on a chromatogram makes it difficult to decide whether the PCB is being partly degraded, mineralized, adsorbed to the fungal biomass, or bound to glassware, soil particles, or wood chips. Even when experiments with killed-cell and abiotic controls are performed, the extraction efficiency and standard error can make data difficult to interpret. For example, recoveries can range anywhere from 40 to 100% depending on the congener used and the fungus being investigated (17). On the other hand, recovery of significant amounts of 14CO2 from the cultures incubated with a 14C substrate provides definitive proof of fungal metabolism. There appears to be only one report relating data from these two techniques (18), and in that study, [U-14C]Aroclor 1254, rather than an individual congener, was used.
In this study, we examined the ability of 12 white rot fungal strains to metabolize selected PCB congeners to determine which strains were the most active degraders. Included in this group was P. chrysosporium ATCC 24725, a strain used extensively in PCB studies (3, 14, 18, 35, 39, 41–43). Six PCB congeners were selected to give a range of chlorine substitutions and therefore a range of potential biodegradability which was monitored by GC. One of the chosen congeners was 14C labeled and used in studies to compare the results from a mineralization method with those from the GC method.
MATERIALS AND METHODS
Chemicals.
Six PCB congeners, 2,3-dichlorobiphenyl (2,3-DCB), 4,4′-dichlorobiphenyl (4,4′-DCB), 2,4′,5-trichlorobiphenyl (2,4′,5-TCB), 2,2′,4,4′-tetrachlorobiphenyl (2,2′,4,4′-TeCB), 2,2′,5,5′-tetrachlorobiphenyl (2,2′,5,5′-TeCB), and 2,2′,4,4′,5,5′-hexachlorobiphenyl (2,2′,4,4′,5,5′-HCB), and octachloronaphthalene were purchased from Accustandard Inc. (New Haven, Conn.). 2,4′,5-[U-14C]TCB (22 mCi/mmol; >99% purity) was obtained from the Department of Environmental Chemistry, Stockholm University (Stockholm, Sweden). The purity of the 2,4′,5-[U-14C]TCB was determined by the supplier by thin-layer chromatography and radiometric scanning. All other chemicals used in this study were reagent grade or better. Veratryl alcohol was extracted into dichloromethane from aqueous 1 M NaOH to remove the trace contaminant methyl-3-methoxy-4-hydroxybenzoate (40).
Fungi.
The fungi used in this study were from the University of Alberta Microfungus and Herbarium (UAMH) culture collection (L. Sigler, Devonian Botanical Garden, University of Alberta, Edmonton, Alberta, Canada T6G 2E1) and from the American Type Culture Collection (Manassas, Va.). They were B. adusta UAMH 7308 and UAMH 8258; P. ostreatus UAMH 7964, UAMH 7972, UAMH 7988, UAMH 7989, and UAMH 7992; T. versicolor UAMH 8272; P. chrysosporium UAMH 3641, UAMH 3642, and ATCC 24725; and Sporotrichum pruinosum UAMH 8272. All cultures were incubated at 28°C, with the exception of P. chrysosporium strains, which were incubated at 37°C. Working slants of each fungus were grown on potato dextrose agar (Difco, Detroit, Mich.) for 1 week and then stored at 4°C.
Culture preparation.
Each organism was grown on potato dextrose agar plates prior to inoculation into 200 ml of a liquid nutrient-rich medium in 500-ml Erlenmeyer flasks. Those fungi which tended to grow in pellet form were homogenized in a 50-ml stainless steel homogenizer (Omni-Mixer; Sorvall, Norwalk, Conn.) for 15 s. Growth was for 7 days at 28 or 37°C on a rotary shaker at 200 rpm. Two liquid media were used, one nitrogen deficient and the other nutrient rich. The nitrogen-deficient medium contained, per liter of distilled water, glucose (10 g), KH2PO4 (2 g), MgSO4 · 7H2O (0.5 g), FeSO4 · 7H2O (0.5 mg), MnSO4 · H2O (0.16 mg), ZnSO4 · 7H2O (0.14 mg), and CoCI · 6H2O (0.29 mg). Yeast extract (0.2 g/liter; Difco) and Bacto Peptone (0.1 g/liter; Difco) were added to the medium because the exact nitrogen and vitamin requirements of the fungal strains were unknown. For the nutrient-rich medium, 2% (wt/vol) malt extract was added to the nitrogen-deficient medium. 2,2-Dimethylsuccinic acid (1.46 g) dissolved in 6.9 ml of 1 M NaOH was added to buffer the media at pH 4.5.
After 7 days of incubation, fungi were harvested by centrifugation at 10,000 × g, washed with 100 ml of nitrogen-deficient medium, and homogenized if necessary. Fungal wet weight was determined after centrifuging the culture at 1,800 × g for 10 min and decanting the supernatant.
Biodegradation of a mixture of PCB congeners. (i) Incubation.
Ten milliliters of nitrogen-deficient medium was added to 158-ml serum bottles, sealed with Teflon-lined stoppers (The West Company, Lionville, Pa.), and autoclaved. Each serum bottle received 40 μl of a congener mix containing 2,3-DCB, 4,4′-DCB, 2,4′,5-TCB, 2,2′,4,4′-TeCB, 2,2′,5,5′-TeCB, and 2,2′,4,4′,5,5′-HCB in acetone, giving a final concentration of 10 μg of each congener per ml. Each serum bottle received a 10% (vol/vol) inoculum containing 0.6 g (wet weight) of biomass, and the cultures were incubated at 28 or 37°C on a rotary shaker at 100 rpm. After 5 days, each serum bottle was flushed with filter-sterilized O2 for 5 min at a flow rate of 100 ml/min to maintain vigorous aerobiosis (14, 42). For each experiment, there were three groups of serum bottles: (i) sterile controls that received only medium and PCBs; (ii) killed controls, which received 1 ml of 7% (vol/vol) perchloric acid for determination of PCB binding to biomass; and (iii) the experimental live cultures. Triplicate samples were used, giving nine serum bottles per fungus for each time point.
(ii) Extraction.
Controls and live cultures were extracted after 0, 7, and 21 days of incubation. At the time of extraction, each culture or control received 1 ml of 7% perchloric acid, 30 ml of hexane, and 100 μg of octachloronaphthalene, as an internal standard. Fungal biomass was homogenized in the serum bottles with a Brinkmann homogenizer with an 11-mm blade and 16-cm shaft. The bottles were resealed with Teflon-lined stoppers and shaken at 190 oscillations/min for 48 h on a horizontal shaker at room temperature. If required, 1 g of anhydrous Na2SO4 was added to break any emulsion that formed. Nine hundred microliters was removed from the hexane phase and diluted with 2.1 ml of pure hexane.
(iii) GC analysis.
A Perkin-Elmer 8500 gas chromatograph with an electron capture detector (ECD) was used. The different congeners were separated in a Supelco SPB-1 wide-bore capillary column (15 m by 0.53 mm) with a film thickness of 0.5 μm with ultrapure N2 as the carrier gas. The injection volume was 1 μl, delivered by a Perkin-Elmer AS 8300 autosampler into a flash vaporizing injector port at 250°C. The oven remained at 120°C for 25 min followed by an increase of 10°C/min to 210°C, which was held for 9 min. The detector temperature was 300°C. The peaks were integrated on a Perkin-Elmer Nelson 900 series integrator. The loss of a congener attributed to biodegradation was calculated by subtracting its concentration in the acid-killed controls from its concentration in the corresponding live cultures.
Calibration curves were constructed with each of the six congeners and octachloronaphthalene, and these calibrations verified that the concentrations of the congeners in the diluted culture extracts fell within the linear range of the ECD.
2,4′,5-[U-14C]TCB mineralization assays.
Ten milliliters of nitrogen-deficient medium was added to 158-ml serum bottles, sealed with Teflon-lined stoppers, and autoclaved. Each serum bottle received 0.6 g (wet weight) of biomass, was flushed with O2 for 5 min at a flow rate of 100 ml/min, and was incubated for 5 days at 28 or 37°C (depending upon the inoculum) statically and in the dark. Each experiment contained triplicate sterile controls, acid-killed controls, and live cultures. After 5 days, each serum bottle received a mixture of 100 μg of unlabeled 2,4′,5-TCB and 1 μg of 2,4′,5-[U-14C]TCB in 40 μl of acetone. Cultures were returned to the incubator and after 2, 6, 10, 14, 18, 22, and 30 days were flushed with filter-sterilized O2 to remove 14CO2.
To collect 14CO2, the stopper on a serum bottle was pierced with a 38-mm 18-gauge needle to allow 100 ml of O2 per min to flow through the serum bottles for 10 min. An outlet needle from the serum bottle was connected to a Florisil column described by Dietrich et al. (14) followed by two scintillation vials in series containing 10 ml of aqueous counting scintillant (Amersham Canada Ltd., Oakville, Ontario, Canada) and 1 ml of Carbo-Sorb (Packard Instrumentation Co., Downers Grove, Ill.) to trap the 14CO2. After the serum bottles were flushed, the Teflon-lined stoppers were replaced to prevent the loss of 14CO2 through the pierced holes. The efficiency of the 14CO2 recovery process was determined to be 90% by adding a known amount of NaH14CO3 to sterile medium and then flushing and trapping the radioactivity.
At the end of each 30-day experiment, the medium in the serum bottles was acidified with 1 ml of 7% (vol/vol) perchloric acid and flushed as described above to determine if any H14CO3− remained in the liquid phase. No more than 0.05% of the original radioactivity was recovered as 14CO2, indicating that very little H14CO3− remained in the liquid phase. All 14C counts were determined with a Beckman LS 3801 scintillation counter.
LiP and MnP assays.
Nitrogen-deficient medium (10 ml) was added to 158-ml serum bottles, sealed with Teflon-lined stoppers, and autoclaved. Each serum bottle received a 10% (vol/vol) inoculum containing 0.8 g (wet weight) of biomass and was then flushed with O2 for 5 min at a flow rate of 100 ml/min and incubated at 28 or 37°C (depending upon the inoculum) statically, in the dark for 7 days. Each fungal culture was grown in triplicate. After 7 days of incubation, triplicate cultures were combined and centrifuged at 1,800 × g for 10 min and the peroxidase activities were determined on the supernatant.
LiP activity was determined by the method of Tien and Kirk (40) with veratryl alcohol as the substrate. MnP activity was determined by the method of Kuwahara et al. (29) with phenol red as the substrate. Commercial preparations of partly purified LiP and MnP were purchased from Intech One-Eighty Corp. (North Logan, Utah). Activity measurements of these preparations were consistent with the values provided by the supplier.
RESULTS
GC analysis of fungal PCB biodegradation.
The recovery of PCB congeners from fungal cultures can be affected by (i) fungal biodegradation, (ii) binding to fungal biomass, (iii) binding to glassware, and (iv) abiotic losses during extraction and sample preparation. Mean abiotic losses ranged from (3.4 ± 11)% for 2,2′,4,4′-TeCB to (17 ± 6.7)% for 2,2′,4,4′,5,5′-HCB, which are similar to those reported by others (14, 41).
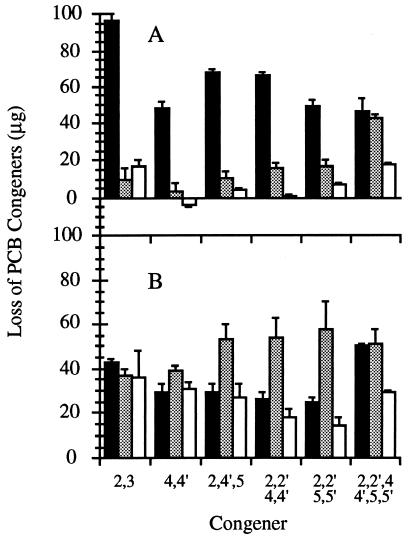
For B. adusta, the losses of the six congeners from the sterile controls ranged from 1 to 18% (Fig. 1A), indicating recoveries of 99 to 82%. Losses of the six congeners from the acid-killed controls were higher, ranging from 3 to 43%. These losses were attributed to congener binding to fungal biomass and abiotic losses. Except for 2,2′,4,4′,5,5′-HCB, the congener losses were significantly greater (P < 0.05) in the live cultures than in the controls, ranging from 46 to 97%. These differences were attributed to biodegradation.
FIG. 1.
Removal of six PCB congeners over 21 days from live cultures (▪) of B. adusta UAMH 8258 (A) and P. chrysosporium ATCC 24725 (B), acid-killed controls (░⃞), and abiotic, cell-free controls (□). Each triplicate serum bottle initially received 100 μg of 2,3-DCB, 4,4′-DCB, 2,4′,5-TCB, 2,2′,4,4′-TeCB, 2,2′,5,5′-TeCB, and 2,2′,4,4′,5,5′-HCB.
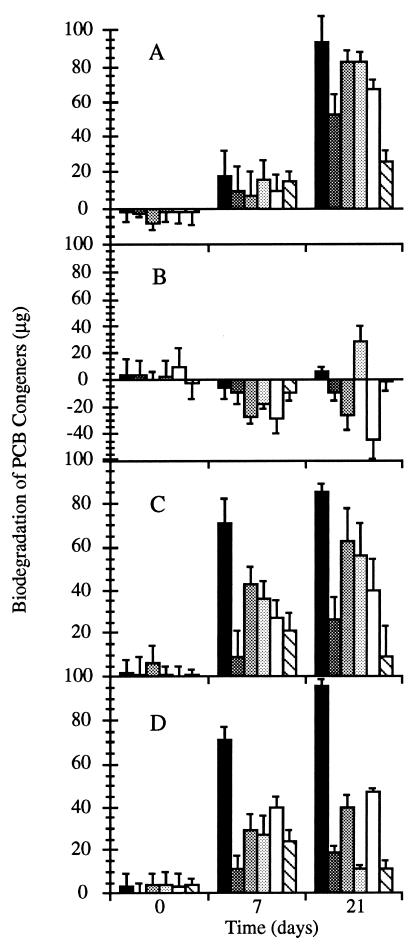
B. adusta UAMH 7308 degraded all six congeners (Fig. 2A): over 60% of 2,3-DCB, 2,4′,5-TCB, 2,2′,4,4′-TeCB, and 2,2′,5,5′-TeCB; over 40% of 4,4′-DCB; and 20% of 2,2′,4,4′,5,5′-HCB were degraded. Congener degradation by UAMH 8258 (Fig. 1A) was comparable to that by B. adusta UAMH 7308 at 21 days, but the former strain was more active in the first week, when it degraded 60% of the 2,3-DCB, 20% of the 2,2′,4,4′,5,5′-HCB, and 40% of each of the other four congeners (data not shown). This is the first time that a Bjerkandera species has been shown to be capable of degrading PCBs.
FIG. 2.
Biodegradation of six PCB congeners, 2,3-DCB (▪), 4,4′-DCB (▩) 2,4′,5-TCB (░⃞), 2,2′,4,4′-TeCB ( ), 2,2′,5,5′-TeCB (□), and 2,2′,4,4′,5,5′-HCB (▧), by B. adusta UAMH 7308 (A), P. chrysosporium ATCC 24725 (B), P. ostreatus UAMH 7972 (C), and T. versicolor UAMH 8272 (D) incubated for 21 days with 100 μg of each congener. Biodegradation is the difference between the average congener removal from live cultures and that in the controls killed by acid addition on day 0.
For P. chrysosporium ATCC 24725, the major removal mechanism was binding of PCBs to fungal biomass (Fig. 1B). This was most apparent after the acid-killed control removal values were subtracted from the removal values for the live cultures. After this calculation, the losses due to biodegradation were small, nonexistent, or negative (Fig. 2B). Two other strains of P. chrysosporium (UAMH 3641 and UAMH 3642) produced data similar to that from ATCC 24725.
All four P. ostreatus strains tested degraded the di-, tri-, and tetrachlorobiphenyl congeners extensively and to similar degrees (Fig. 2C). T. versicolor extensively degraded 2,3-DCB but was less effective with other congeners (Fig. 2D).
2,4′,5-[U-14C]TCB mineralization.
2,4′,5-TCB was chosen for mineralization studies because of its availability. The most active organism was T. versicolor, which mineralized 11% of the 2,4′,5-[U-14C]TCB to 14CO2 over 22 days, with 10% mineralized in the first 14 days (Table 1). The next most active fungus, B. adusta UAMH 8258, mineralized 6.9% of the 2,4′,5-TCB over 22 days. All the P. chrysosporium strains mineralized between 1.2 and 3.0% of the 2,4′,5-[U-14C]TCB (data not shown).
TABLE 1.
Mineralization of 2,4′,5-[U-14C]TCB at 22 days
| Organism |
14CO2 produced as
% of total 14C added to cultures
|
||
|---|---|---|---|
| Live culture | Acid-killed control | Sterile control | |
| Bjerkandera adusta UAMH 8258 | 6.9 ± 3.3 | 0.25 ± 0.13 | 0.16 ± 0.06 |
| Bjerkandera adusta UAMH 7308 | 4.9 ± 1.1 | 0.17 ± 0.06 | 0.16 ± 0.06 |
| Pleurotus ostreatus UAMH 7964 | 4.2 ± 0.3 | 0.29 ± 0.05 | 0.18 ± 0.06 |
| Phanerochaete chrysosporium ATCC 24725a | 1.9 ± 1.6 | 0.17 ± 0.04 | 0.21 ± 0.05 |
| Trametes versicolor UAMH 8272 | 11 ± 0.3 | 0.15 ± 0.01 | 0.16 ± 0.06 |
Also called strain BKM F-1767.
LiP and MnP activity.
We detected low levels of LiP and MnP from most of the fungi examined. No LiP activity was detected in B. adusta UAMH 7308 and T. versicolor, while the P. chrysosporium strains had no MnP activity, as expected (9). B. adusta UAMH 8258 and all the P. ostreatus strains had both LiP and MnP activities.
We determined if biodegradation and mineralization were correlated. Table 2 presents data on the biodegradation of 2,3-DCB, which was the congener degraded by most fungal isolates, along with data on the biodegradation of 2,4′,5-TCB and mineralization of 2,4′,5-[U-14C]TCB to allow direct comparison of biodegradation and mineralization. Data from this table was used for regression analyses to relate mineralization to biodegradation and enzyme activity. In all cases, the correlation coefficients were less than 0.67, where 0.58 is the lower limit for significance (P = 0.05) for 12 data points giving 10 degrees of freedom (36), and typically, the coefficients were around 0.3. Thus, the regression analyses indicated that the biodegradation of these congeners was not an indication of the ability of the fungus to mineralize the PCB congener and likely does not depend on the presence of either of the peroxidases.
TABLE 2.
Biodegradation of 2,3-DCB and 2,4′,5-TCB and net mineralization of 2,4′,5-[U-14C]TCB in comparison to LiP and MnP activity of each organism
| Organism | Net mineralizationa of 2,4′,5[U-14C]TCB (%) | Biodegradationb (μg)
of:
|
Activity (U/liter) of:
|
||
|---|---|---|---|---|---|
| 2,3-DCB | 2,4′,5-TCB | LiPc | MnPd | ||
| B. adusta UAMH 8258 | 6.7 | 87 | 57 | 0.03 | 1.2 |
| B. adusta UAMH 7308 | 4.8 | 93 | 80 | NDe | 7.5 |
| P. ostreatus UAMH 7964 | 3.9 | 67 | 51 | 0.15 | 5.0 |
| P. chrysosporium ATCC 24725 | 1.7 | 6 | −27 | 0.39 | ND |
| T. versicolor UAMH 8272 | 11 | 96 | 40 | ND | 3.9 |
Net mineralization is the difference between the average cumulative 14CO2 produced in the live cultures and that in the acid-killed controls after 22 days of incubation.
Biodegradation is the difference between the average congener removal in the live cultures and that in the acid-killed controls after 21 days of incubation. Initially, 100 μg of each congener was present.
One enzyme unit (U) represents 1 μmol of veratryl alcohol (molar extinction coefficient = 9,300 M−1 cm−1; Intech One-Eighty Corp.) oxidized to veratraldehyde per min at pH 2.5 and 25°C.
One unit represents 1 μmol of phenol red oxidized per min at pH 4.5 and 25°C. The molar extinction coefficient of phenol red is 4,460 M−1 cm−1 (31).
ND, not detected.
DISCUSSION
Eight fungal strains used in this study degraded the six congeners more actively than did any of the P. chrysosporium strains examined, including ATCC 24725, a strain that is frequently used in fungal PCB biodegradation studies (14, 18, 39, 42, 43). B. adusta UAMH 8258 and UAMH 7308 were the most active degraders of PCB congeners, based on GC and mineralization methods. T. versicolor actively degraded 2,3-DCB but was less active in degrading the congener used for mineralization, 2,4′,5-TCB (40%), even though this fungus showed the highest mineralization (11%). P. ostreatus UAMH 7964 was also active in both mineralization and biodegradation, while the four other P. ostreatus strains were active only in degradation.
Yadav et al. (42) used ATCC 24725 for congener-specific analysis of the degradation of Aroclor 1242, a mixture of congeners with 42% chlorine by weight and an average of three chlorines per biphenyl. They found that 89, 54, and 47% of 2,3-DCB, 2,4′,5-TCB, and 2,2′,4,4′-TeCB, respectively, were degraded over a 30-day period. In our study, B. adusta UAMH 7308 degraded 93, 82, and 82% of 2,3-DCB, 2,4′,5-TCB, and 2,2′,4,4′-TeCB, respectively, over a 21-day period, suggesting that B. adusta may degrade Aroclor 1242 more extensively than does P. chrysosporium.
PCB biodegradation was difficult to document because of congener binding to biomass and glassware. This problem was particularly severe with the strains of P. chrysosporium. Other researchers also have encountered this problem. For example, Dietrich et al. (14) found that up to 60% of 4,4′-DCB was adsorbed to the biomass and glassware during the first 12 days of incubation and that >60% of the 3,3′,4,4′-TeCB and 2,2′,4,4′,5,5′-HCB was bound to the biomass. We found increased extraction efficiencies when new serum bottles sealed with Teflon liners were used and when extractions were performed in the serum bottles directly without transferring the supernatant to separatory funnels.
The choice of PCB congeners was based primarily on the criteria of Bedard et al. (7) for bacterial studies of PCB biodegradation. The pattern of degradation of specific congeners by bacteria has been well established previously (4, 7), and the evidence of a similar pattern, with respect to chlorine content, has been shown for fungi (14, 39, 41, 42). Congener biodegradation in our study was similar to that reported by others (17, 43), except for T. versicolor UAMH 8272. This strain could effectively degrade 2,3-DCB (96%) but was much less effective on all the other congeners (Fig. 2D). Thus, different fungal species attack congeners differently.
Mineralization results are difficult to compare between studies unless the same radiolabeled congener is used. The availability of a radiolabeled congener determined which congener we used. Mineralization of individual congeners depends on the chlorine content of the congener and the fungal strain used (14, 39, 41). Dietrich et al. (14) and Thomas et al. (39) observed that mineralization decreases as chlorine content increases. With P. chrysosporium, mineralization of the following congeners has been observed over approximately 30 days (percent mineralization in parentheses): 2-chlorobiphenyl (up to 16%), 4,4′-DCB (11%), 2,2′,4,4′-TeCB (up to 10%), 3,3′,4,4′-TeCB (up to 1.4%), and 2,2′,4,4′,5,5′-HCB (<1%) (11, 14, 39, 41). Based on these observations, we expected 1.4 to 11% of the 2,4′,5-TCB to be mineralized. All strains we examined gave results in the predicted range (Table 2).
We are unaware of other studies in which microbial degradation of a selected congener was evaluated by both GC and radiorespirometric methods. We obtained different results with each method (Table 2), and these results were not correlated. For example, four P. ostreatus strains degraded between 53 and 77 μg of the 100 μg of 2,4′,5-TCB based on GC-ECD analyses, but they released <0.5% of the radioactivity from 2,4′,5-[U-14C]TCB as 14CO2 (data not presented). Similarly, the highest mineralization was by T. versicolor, which released 11% of the label as 14CO2, yet by GC-ECD analysis, this strain degraded only 40% of the 2,4′,5-TCB (Table 2), the lowest value of the five fungi, excluding P. chrysosporium. These results imply that different white rot fungi have enzymes of different specificity or different mechanisms for degrading PCB congeners. Detection and identification of polar intermediates and metabolites will require modified analytical procedures, including derivatization of culture extracts prior to analysis by GC-mass spectrometry.
The LiP activities observed in our study (Table 2) were considerably lower than those reported for these fungi by other workers (9, 12, 30, 39). This difference may be due to the yeast extract and peptone included at low levels for those strains with uncertain growth requirements. The production of LiP and MnP from the same strain of P. chrysosporium under nutrient-limited conditions has been documented elsewhere (13, 20, 29). In our study, the P. chrysosporium strains showed LiP activity only, probably due to the low Mn(II) content of the medium (9), whereas all the other fungi exhibited MnP activity (Table 2). The differences in peroxidase activity may be due to different growth characteristics. Kirk et al. (27) found that ligninase activity and the time to reach maximum activity varied among strains of P. chrysosporium. We chose a 7-day incubation period before peroxidase activity measurement because, in many cases, biodegradation (Fig. 2A, C, and D) and mineralization (data not shown) of the congeners started within 7 days.
The observation that the peroxidase levels were not related to PCB degradation could, perhaps, have been predicted since PCBs have ionization potentials around 9 eV, higher than the upper limit for LiP-catalyzed reactions, which is around 8 eV (24), and since the related compound dichlorodiphenyltrichloroethane is not a substrate for LiP (28). Regression analysis of biodegradation, mineralization, and LiP and MnP activity for all 12 fungal strains revealed that no linear correlation existed. Thomas et al. (39) also concluded that enzymes other than ligninases and Mn-dependent peroxidase must be involved in the oxidation of PCBs. It may be that other enzymes related to lignin degradation, such as laccase or aryl alcohol oxidase (2), or combinations of enzymes are involved.
There are advantages and disadvantages to using the GC or radiorespirometric method to study the microbial degradation of PCB congeners. The GC method is more versatile, allowing many congeners to be monitored during a single experiment, whereas using a 14C-labeled congener allows the fate of only a single congener to be monitored in an experiment. The loss of a congener from a GC chromatogram indicates that the molecule was transformed to products that are not amenable to detection under a given set of analytical conditions but gives no clue of the extent to which the congener was transformed. In contrast, the detection of 14CO2 clearly indicates that at least part of the molecule was extensively degraded. Using both methods together gives a measure of the overall removal of the congener and the amount of the substrate that can be mineralized. From our experience, the amount of biodegradation of 2,4′,5-TCB measured by GC analyses was greater than the amount of its mineralization (Table 2). Because adsorption of the congeners to fungal biomass can be a major mechanism of removal, the inclusion of acid-killed controls is essential for biodegradation studies with GC analyses. These controls are less critical for mineralization studies in which 14CO2 is readily released from the cultures. The major limitation of the radiorespirometric method is that very few of the 209 congeners are readily available as 14C-labeled compounds from commercial sources.
Regardless of the differences in absolute values between different studies for PCB biodegradation and mineralization by fungi, many of which can be explained by methodological differences, the data described in this report consistently showed that P. chrysosporium ATCC 24725 and the other P. chrysosporium strains were among the poorest of the white rot fungi in their ability to degrade PCBs. Our killed controls showed that congener binding to the P. chrysosporium biomass was a major factor in the low recovery of congeners in our experiments. The low PCB metabolizing activity demonstrated by these P. chrysosporium strains might be due to their long-term preservation in culture collections. Most of the fungal strains exhibiting high activity in our study do not have a long association with culture collections.
ACKNOWLEDGMENTS
This work was supported by a grant from the Natural Science and Engineering Research Council of Canada.
We thank D. C. Villeneuve, Health Protection Branch, Health Canada, for the loan of the Perkin-Elmer GC-ECD, and Åake Bergman, Stockholm University, for supplying the radiolabeled congener. We also thank Atsumi Hashimoto for storing and handling the fungi and Paola Marcato for assistance with enzyme assays.
REFERENCES
- 1.Abramowicz D A. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–251. [Google Scholar]
- 2.Ander P, Marzullo L. Sugar oxidoreductases and veratryl alcohol oxidase as related to lignin degradation. J Biotechnol. 1997;53:115–131. doi: 10.1016/s0168-1656(97)01680-5. [DOI] [PubMed] [Google Scholar]
- 3.Aust S D. Degradation of environmental pollutants by Phanerochaete chrysosporium. Microb Ecol. 1990;20:197–209. doi: 10.1007/BF02543877. [DOI] [PubMed] [Google Scholar]
- 4.Bedard D L, Haberl M L. Influence of chlorine substitution pattern on the degradation of polychlorinated biphenyls by eight bacterial strains. Microb Ecol. 1990;20:87–102. doi: 10.1007/BF02543870. [DOI] [PubMed] [Google Scholar]
- 5.Bedard D L, Haberl M L, May R J, Brennan M J. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophusH850. Appl Environ Microbiol. 1987;53:1103–1112. doi: 10.1128/aem.53.5.1103-1112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 127–216. [Google Scholar]
- 7.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedard D L, Wagner R E, Brennan M J, Haberl M L, Brown J F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophusH850. Appl Environ Microbiol. 1987;53:1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnarme P, Jeffries T W. Mn(II) regulation of lignin peroxidase and manganese-dependent peroxidases from lignin-degrading white rot fungi. Appl Environ Microbiol. 1990;56:210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J F J, Wagner R E, Feng H, Bedard D L, Brennan M J, Carnahan J C, May R J. Environmental dechlorination of PCBs. Environ Toxicol Chem. 1987;6:579–593. [Google Scholar]
- 11.Bumpus J A, Tien M, Wright D, Aust S D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985;228:1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- 12.Collins P J, Field J A, Teunissen P, Dobson A D W. Stabilization of lignin peroxidase in white rot fungi by tryptophan. Appl Environ Microbiol. 1997;63:2543–2548. doi: 10.1128/aem.63.7.2543-2548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta A, Bettermann A, Kirk T K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporiumduring wood decay. Appl Environ Microbiol. 1991;57:1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich D, Hickey W J, Lamar R. Degradation of 4,4′-dichlorobiphenyl, 3,3′,4,4′-tetrachlorobiphenyl, and 2,2′,4,4′,5,5′-hexachlorobiphenyl by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:3904–3909. doi: 10.1128/aem.61.11.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dmochewitz S, Ballschmiter K. Microbial transformation of technical mixtures of polychlorinated biphenyls (PCB) by the fungus Aspergillus niger. Chemosphere. 1988;17:111–121. [Google Scholar]
- 16.Dodge R H, Cerniglia C E, Gibson D T. Fungal metabolism of biphenyl. Biochem J. 1979;178:223–230. doi: 10.1042/bj1780223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly P K, Fletcher J S. PCB metabolism by ectomycorrhizal fungi. Bull Environ Contam Toxicol. 1995;54:507–513. doi: 10.1007/BF00192592. [DOI] [PubMed] [Google Scholar]
- 18.Eaton D C. Mineralization of polychlorinated biphenyls by Phanerochaete chrysosporium: a ligninolytic fungus. Enzyme Microb Technol. 1985;7:194–196. [Google Scholar]
- 19.Erickson M D. Analytical chemistry of PCBs. Boca Raton, Fla: Lewis Publishers; 1991. [Google Scholar]
- 20.Farrell R L, Murtagh K E, Tien M, Mozuch M D, Kirk T K. Physical and enzymatic properties of lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Enzyme Microb Technol. 1989;11:322–328. [Google Scholar]
- 21.Furukawa K. Microbial degradation of polychlorinated biphenyls (PCBs) In: Chakrabarty A M, editor. Biodegradation and detoxification of environmental pollutants. Boca Raton, Fla: CRC Press, Inc.; 1982. pp. 33–57. [Google Scholar]
- 22.Gibson D T, Cruden D L, Haddock J D, Zylstra G J, Brand J M. Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenesKF707. J Bacteriol. 1993;175:4561–4564. doi: 10.1128/jb.175.14.4561-4564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glenn J K, Gold M H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;242:329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- 24.Kersten P J, Kalyanaraman B, Hammel K E, Reinhammar B. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990;268:475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keyser P, Kirk T K, Zeikus J G. Ligninolytic enzyme system of Phanerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978;135:790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirk T K, Schultz E, Connors W J, Lorenz L F, Zeikus J G. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol. 1978;117:277–285. [Google Scholar]
- 27.Kirk T K, Tien M, Johnsrud S C, Eriksson K-E. Lignin degrading activity of Phanerochaete chrysosporiumBurds.: comparison of cellulase-negative and other strains. Enzyme Microb Technol. 1986;6:75–80. [Google Scholar]
- 28.Köhler A, Jäger A, Willershausen H, Graf H. Extracellular ligninase of Phanerochaete chrysosporiumBurdsall has no role in the degradation of DDT. Appl Microbiol Biotechnol. 1988;29:618–620. [Google Scholar]
- 29.Kuwahara M, Glenn J K, Morgan M A, Gold M H. Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 1984;169:247–250. [Google Scholar]
- 30.Mester T, Pena M, Field J A. Nutrient regulation of extracellular peroxidases in the white rot fungus Bjerkanderasp. strain BOS55. Appl Microbiol Biotechnol. 1996;44:778–784. [Google Scholar]
- 31.Michel F C, Dass S B, Grulke E A, Reddy A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991;57:2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murado M A, Tejedor M C, Baluja G. Interactions between polychlorinated biphenyls (PCBs) and soil microfungi. Effects of Aroclor-1254 and other PCBs on Aspergillus flavuscultures. Bull Environ Contam Toxicol. 1976;15:768–774. doi: 10.1007/BF01685631. [DOI] [PubMed] [Google Scholar]
- 33.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quensen J F, III, Tiedje J M, Boyd S A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988;242:752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- 35.Sasek V, Volfova O, Erbanova P, Vyas B R M, Matucha M. Degradation of PCBs by white rot fungi, methylotrophic and hydrocarbon utilizing yeasts and bacteria. Biotechnol Lett. 1993;15:521–526. [Google Scholar]
- 36.Steel R G D, Torrie J H. Principles and procedures of statistics. A biometric approach. 2nd ed. New York, N.Y: McGraw-Hill; 1980. [Google Scholar]
- 37.Sutherland J B, Rafii F, Kahn A A, Cerniglia C E. Mechanism of polycyclic aromatic hydrocarbon metabolism. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 269–306. [Google Scholar]
- 38.Tejedor M C, Murado M A, Baluja G. Oxidative metabolism in Saccharomyces cerevisiaeas affected by polychlorinated biphenyls. Bull Environ Contam Toxicol. 1979;22:439–448. doi: 10.1007/BF02026968. [DOI] [PubMed] [Google Scholar]
- 39.Thomas D R, Carswell K S, Georgiou G. Mineralization of biphenyl and PCBs by the white rot fungus Phanerochaete chrysosporium. Biotechnol Bioeng. 1992;40:1395–1402. doi: 10.1002/bit.260401114. [DOI] [PubMed] [Google Scholar]
- 40.Tien M, Kirk T K. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161:238–249. [Google Scholar]
- 41.Vyas B R M, Sasek V, Matucha M, Bubner M. Degradation of 3,3′,4,4′-tetrachlorobiphenyl by selected white rot fungi. Chemosphere. 1994;28:1127–1134. [Google Scholar]
- 42.Yadav J S, Quensen III J F, Tiedje J M, Reddy C A. Degradation of polychlorinated biphenyl mixtures (Aroclors 1242, 1254, and 1260) by the white rot fungus Phanerochaete chrysosporiumas evidenced by congener-specific analysis. Appl Environ Microbiol. 1995;61:2560–2565. doi: 10.1128/aem.61.7.2560-2565.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeddel A, Majcherczyk A, Huttermann A. Degradation of polychlorinated biphenyls by white rot fungi Pleurotus ostreatus and Trametes versicolorin a solid state system. Toxicol Environ Chem. 1993;40:225–266. [Google Scholar]