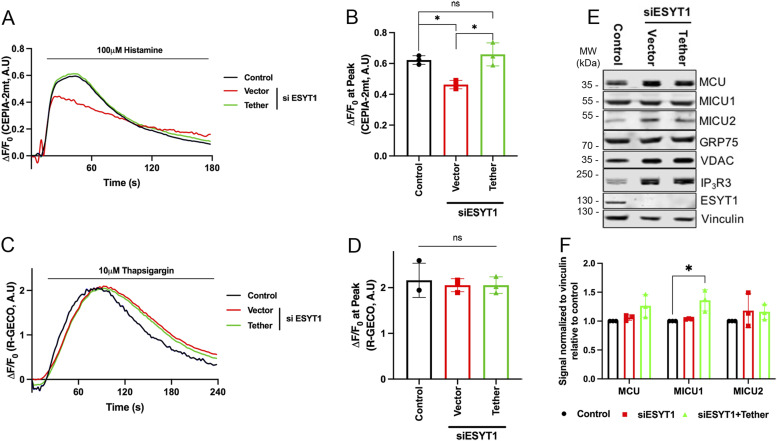
Figure 5. ESYT1 is required for ER to mitochondria Ca2+ transfer in Hela cells.
(A) Trace of mitochondrial (Ca2+) upon histamine stimulation (100 μM) in control HeLa cells, cells knocked-down for ESYT1, and cells knocked-down for ESYT1 that express an artificial ER–mitochondria tether. All cells express the mitochondrial Ca2+ probe, CEPIA-2mt. (B) Quantification of the maximal fluorescence intensity fold-change (ΔF/F0) of CEPIA-2mt induced by histamine. Results are expressed as mean ± SD; From >50 cells per condition; n = 3 independent experiments. ns: not significant; *P < 0.05 (Turkey’s multiple comparisons test). (C) Trace of cytosolic (Ca2+) upon thapsigargin treatment (10 μM) in control HeLa cells, cells knocked-down for ESYT1 and cells knocked-down for ESYT1 that express an artificial ER–mitochondria tether. All cells express the cytosolic Ca2+ probe, R-GECO. (D) Quantification of the maximal fluorescence intensity fold change (ΔF/F0) of R-GECO upon thapsigargin treatment. Results are expressed as mean ± SD; from >50 cells per condition; n = 3 independent experiments. ns: not significant (Turkey’s multiple comparisons test). (E) Whole-cell lysates of control HeLa cells, cells knocked-down for ESYT1 and cells knocked-down for ESYT1 that express an artificial ER–mitochondria tether were analyzed by SDS–PAGE and immunoblotting. Vinculin was used as a loading control. (E, F) Quantification of three independent experiments as in panel (E). The graphs show the signal normalized to vinculin relative to control. Results are expressed as means ± S.D. Two-way ANOVA with a Dunnett correction for multiple comparisons was performed. *P < 0.05.