Abstract
Background
Acute decompensated aortic stenosis (ADAS) is common and associated with higher mortality, acute kidney injury (AKI) and longer hospital length of stay (LoS) compared with electively treated stable AS. The aim of this study was to assess the impact of a dedicated pathway that reduces time to transcatheter aortic valve implantation (TAVI) in ADAS, hypothesizing that LoS can be reduced without compromising patient safety.
Methods and results
Using a prospective, open label, cluster design, patients from 5 referring centres were allocated to the ASessment and TReatment In Decompensated Aortic Stenosis (ASTRID-AS) pathway where the diagnosis, referral, investigations and treatment of ADAS were prioritised and expedited. 15 hospitals remained on the conventional pathway that followed the same process, albeit according to a waiting list. The primary efficacy endpoint was hospital LoS and the secondary safety endpoint, a composite of death or AKI at 30 days post-TAVI. 58 conventional patients and 25 ASTRID-AS patients were included in this study. Time to TAVI in the conventional vs. ASTRID-AS cohort was 22 (15–30) vs. 10 (6–12) days; P < 0.001, respectively. Length of hospital stay was 24 (18–33) vs. 13 (8–18) days; P < 0.001, respectively. 13.4 bed days were saved per patient using the ASTRID-AS pathway. Secondary safety endpoint occurred in 12 (20.7%) vs. 1 (4.0%) patients; P = 0.093, respectively. Procedural complications were similar between the two cohorts.
Conclusion
A dedicated pathway for ADAS that shortens time to TAVI demonstrated reduced hospital LoS without compromising patient safety and a trend towards improving clinical outcomes.
Keywords: Decompensated aortic stenosis, Urgent TAVI, Transcatheter aortic valve replacement, Acute heart failure, Aortic stenosis
Graphical Abstract
Graphical Abstract.
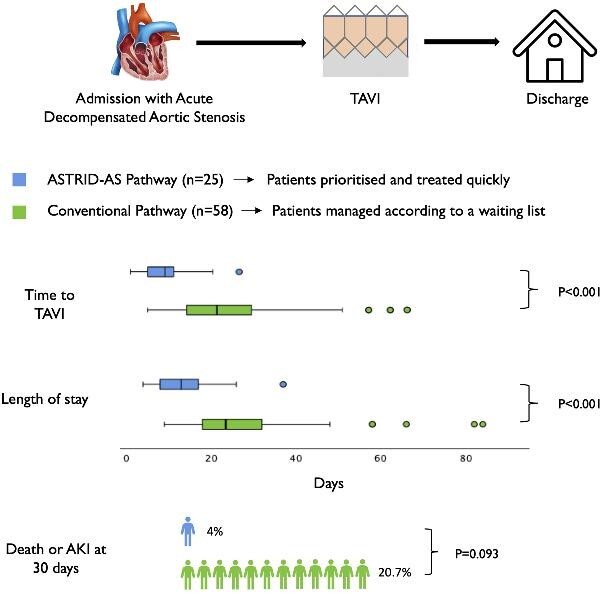
Summary of the ASsessment and TReatment In Decompensated Aortic Stenosis (ASTRID-AS) study. A dedicated pathway, ASTRID-AS, reduced time to TAVI and hospital length of stay in patients with acute decompensated aortic stenosis. There was also a non-significant trend towards lower all-cause mortality and acute kidney injury. The vector image of the heart was obtained from www.vecteezy.com.
Introduction
Up to 1 in 3 patients having a transcatheter aortic valve implantation (TAVI) present acutely with dyspnoea at rest or syncope, termed acute decompensated aortic stenosis (ADAS).1–4,15–16 ADAS patients are often treated medically before having an urgent or elective aortic valve replacement. Compared with elective TAVI for stable aortic stenosis, ADAS patients have a higher mortality at 30 days, higher rate of acute kidney injury (AKI) and longer hospital length of stay (LoS).1,3,4 This has significant implications for patients and healthcare provision. Whilst waiting for definitive treatment in the form of a valve replacement, the heart is still subjected to increased afterload, susceptible to arrhythmias, organs such as the kidneys are prone to under-perfusion and whilst the patient remains admitted in hospital, they are susceptible to nosocomial infections and physical deconditioning.5,6 Two retrospective observational studies on ADAS patients found that delayed treatment with TAVI was associated with an increased mortality rate at 1 and 2 years.7,8 Reducing the time to TAVI could reduce overall hospital LoS and potentially improve outcomes by alleviating the underlying cause of decompensation. Reducing LoS can also improve clinical workflow, reduce healthcare costs and from a patient's perspective, enable quick discharge. However, this needs to be balanced with performing a TAVI in patients with fluid overload, haemodynamic instability, and administering contrast from CT angiography and TAVI in close succession. Therefore a pathway that expedites patient treatment needs to firstly ensure that it does not increase procedural complications or compromise patient safety. This pilot study tests the hypothesis that rapid assessment and treatment of ADAS using TAVI, can reduce hospital LoS, without adversely affecting patient safety and improves clinical outcomes.
Methods
Study design
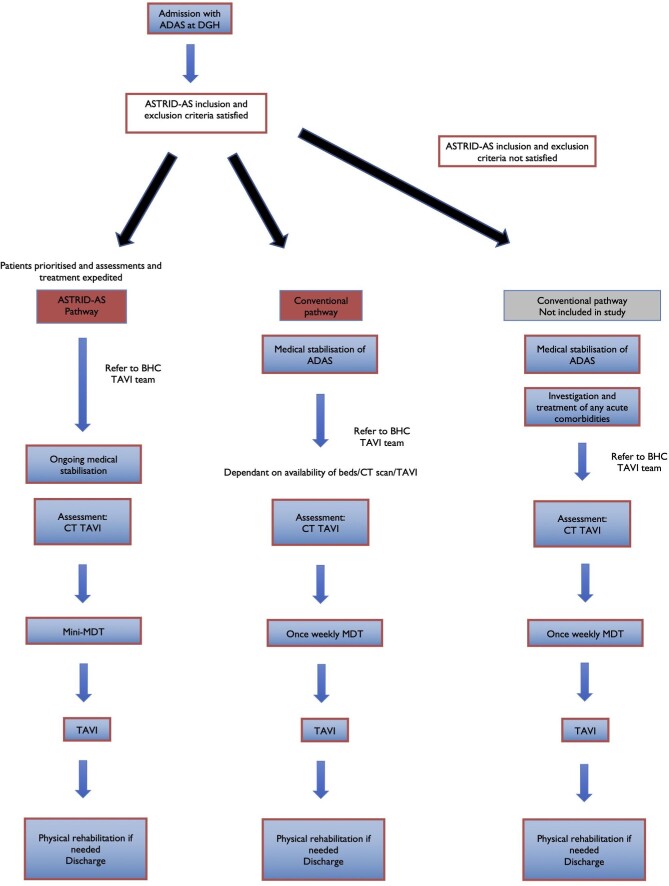
This was a prospective, open label, non-randomised, cluster study conducted in two stages: between October 2019 and March 2020 and November 2021 to March 2022. Due to the COVID pandemic, normal clinical services were disrupted, and data collection was halted from March 2020 to November 2021. We operated two clinical pathways at Barts Heart Centre (BHC)- a tertiary cardiac centre. The first- ASsessment and TReatment In Decompensated Aortic Stenosis (ASTRID-AS) pathway aimed to quickly identify, refer, investigate, and treat ADAS by prioritising the patient and expediting every stage of the process (Figure 1). The second- conventional pathway acted as the control cohort and patients with ADAS underwent the same process but did so according to a waiting list for investigations and TAVI. These patients were medically stabilised, often requiring diuresis for fluid overload before being referred for a TAVI (Figure 1). Details regarding each pathway are in the supplemental methods. Individual referring hospitals that are part of our hospital trust (n = 5) were selected for the ASTRID-AS pathway, whilst the remaining hospitals (n = 15) were selected for the conventional pathway (supplemental methods).
Figure 1.
Clinical pathways for acute decompensated aortic stenosis (ADAS) at Barts Heart Centre. ADAS patients that fulfilled the ASTRID-AS criteria were included in the ASTRID-AS pathway or the conventional pathway depending on which hospital they were initially admitted to. These patients were included in this study. Patients that did not satisfy the ASTRID-AS criteria were treated after medical stabilisation and investigation/treatment of any acute comorbidities. These patients were not included in this study.
Study inclusion and exclusion criteria
For this study we included consecutive patients > 65 years old who were deemed to have ADAS that required admission and urgent TAVI. We excluded patients who needed emergent TAVI at presentation because of cardiogenic shock, non-ST elevation myocardial infarction, Rockwood clinical frailty score > 59 or any preclusion for an urgent TAVI (supplemental methods). These criteria were chosen for the ASTRID-AS pathway to enable treatment with TAVI at the earliest opportunity and early discharge post-TAVI. In order to provide an appropriate and similar control cohort, we choose consecutive patients that fulfilled the ASTRID-AS criteria but were treated using the conventional pathway. Patients that did not fulfil the criteria above were not included in this study (Figure 1). These patients continued to have assessment and treatment in hospital, including a TAVI according to a waiting list.
Endpoints, definitions, inclusion, and exclusion criteria
The primary efficacy end-point was hospital length of stay. This was selected as it allowed us to test our hypothesis in a smaller number of patients compared with if clinical endpoints such as mortality were selected. Secondly, expediting investigations and treatment in ADAS patients could result in AKI or pulmonary oedema due to the increased contrast and volume administered respectively, in a short time period, resulting in longer hospital LoS. As a novel pathway, ensuring that patient safety is not compromised is key before widespread dissemination. The secondary safety endpoint was a composite of stage 2 or greater AKI and all-cause mortality at 30 days post-TAVI. Stage 2 AKI was considered as part of the composite endpoint rather than stage 1 AKI, because the latter is common (up to 22% of stable TAVI patients) and is not associated with an increased risk of mortality compared with no AKI among TAVI populations. However, stage 2 AKI is associated with a 2.5 fold increased risk of mortality at 1 year.10,11 Additional secondary endpoints were procedural complications defined by the VARC 2 criteria.12 ADAS was defined as syncope, acute pulmonary oedema or acute development of dyspnoea at rest (New York Heart Association class 4) caused by severe AS.1,3 AKI was defined as stage 1: estimated glomerular filtration rate (eGFR) decrease > 25% or 150–200% increase in creatinine; stage 2: eGFR decrease > 50% or 201–300% increase in creatinine or stage 3: eGFR decrease > 75% or > 300% increase in creatinine up to 7 days compared with baseline renal function. This was based on the RIFLE and VARC 3 criteria.13,14 Hospital length of stay was calculated from admission at the referring hospital to either in-hospital death or discharge from BHC or a local hospital/rehabilitation centre (if a patient was repatriated for further medical care/rehabilitation), whichever was later. Other definitions are listed in the supplemental methods.
Data collection
All data was prospectively collected onto a dedicated database. Echocardiographic data was obtained from the referring hospital or when the patient arrived at BHC. Mortality data was obtained retrospectively by searching a national database that is updated in real-time (NHS spine).
Ethics
This study was based on a service evaluation project conducted at Barts Heart Centre. Ethical approval was obtained for this study from the Health Research Authority and Health and Care Research Wales (REC reference: 21/NW/0182). The need for informed consent was waived given the clinical rather than research basis of this study. The raw data is not available due to confidentiality purposes.
Transcatheter aortic valve implantation procedure
All patients underwent echocardiography and gated cardiac CT angiography used to plan the TAVI procedure. Each patient was discussed at a multi-disciplinary team meeting. All TAVIs were performed by experienced clinicians at BHC, with the choice of valve technology left to the operator's discretion.
Statistical analysis
We estimated a sample size of 80 patients would be sufficient for this study (supplementary methods). Data is presented as mean ± standard deviation, frequency (percentages), or medians (interquartile range). Inter-group comparisons were performed on baseline characteristics, procedural complications and the primary efficacy endpoint using χ2 test or Fisher's exact test for categorical data, student's t test for parametric data and the Mann-Whitney U test for non-parametric data. Log rank test and cox regression analysis were used to identify the impact of ASTRID-AS on the secondary safety endpoint. A two-sided P value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS software (version 27).
Results
During the study period, 116 patients with ADAS were referred to BHC for an urgent TAVI. 58 were treated in the conventional pathway, 25 in the ASTRID-AS pathway and 33 were excluded from this study as they did not meet the ASTRID-AS criteria (supplemental results).
Baseline characteristics
Table 1 describes the characteristics of both cohorts. Although there were some numerical differences between both cohorts, most were statistically insignificant. Some aortic valve parameters (peak velocity and gradients) were lower in the ASTRID-AS cohort, whilst aortic valve area was similar between both. No patients in either cohort were in cardiogenic shock on presentation.
Table 1.
Baseline characteristics of both cohorts included in this study.
| Parameter | Conventional (n = 58) | ASTRID-AS (n = 25) |
P value | |
|---|---|---|---|---|
| Age (years) | 82 ± 7 | 84 ± 7 | 0.159 | |
| Male sex | 36 (62.1%) | 11 (44%) | 0.128 | |
| Blood parameters | ||||
| Haemoglobin (g/dL) | 12.6 (11.3–13.7) | 11.9 (10.5–13.1) | 0.056 | |
| Estimated GFR (ml/min/1.72 m2) | 64 ± 17 | 71 ± 18 | 0.096 | |
| Albumin (g/L) | 40 (37–43) | 40 (37–44) | 0.709 | |
| Comorbidities | ||||
| Hypertension | 45 (77.6%) | 15 (60.0%) | 0.101 | |
| Hyperlipidaemia | 34 (58.6%) | 14 (56%) | 0.824 | |
| Ischemic heart disease | 19 (32.8%) | 6 (24.0%) | 0.425 | |
| Diabetes Mellitus | 21 (36.2%) | 7 (28%) | 0.468 | |
| Left ventricular systolic dysfunction | 31 (53.4%) | 16 (64%) | 0.374 | |
| Chronic kidney disease | 23 (39.7%) | 6 (24%) | 0.17 | |
| Previous stroke | 3 (5.2%) | 4 (16.0%) | 0.19 | |
| Liver disease | 1 (1.7%) | 1 (4.0%) | 0.514 | |
| Active malignancy | 5 (8.6%) | 0 (0.0.%) | 0.316 | |
| Pulmonary disease | 14 (24.1%) | 2 (8.0%) | 0.13 | |
| Peripheral vascular disease | 1 (1.7%) | 2 (8.0%) | 0.214 | |
| Katz index | 3 | 0 (0.0%) | 1 (4.0%) | 0.201 |
| 4 | 0 (0.0%) | 1 (4.0%) | ||
| 5 | 3 (5.4%) | 1 (4.0%) | ||
| 6 | 53 (94.6%) | 22 (88.0%) | ||
| Rockwood CFS | 2 | 2 (3.5%) | 0 (0.0%) | 0.301 |
| 3 | 21 (36.8%) | 5 (20.0%) | ||
| 4 | 22 (38.6%) | 12 (48.0%) | ||
| 5 | 12 (21.1%) | 8 (32.0%) | ||
| Echocardiographic parameters | ||||
| LV EDD (cm) | 4.8 (4.1–5.4) | 4.7 (4.2–5.2) | 0.967 | |
| LV ESD (cm) | 3.7 (2.8–4.1) | 3.3 (2.8–4.1) | 0.957 | |
| IVS (cm) | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | 0.886 | |
| PWT (cm) | 1.1 (0.9–1.3) | 1.2 (1.1–1.3) | 0.459 | |
| LVEF (%) | 53 (3.7–5.5) | 47 (33–55) | 0.542 | |
| TAPSE (cm) | 1.8 (1.5–2.1) | 2.0 (1.6–2.4) | 0.22 | |
| V max (m/s) | 4.3 ± 0.6 | 4.0 ± 0.6 | 0.024 | |
| Mean gradient (mmHg) | 48 ± 14 | 37 ± 13 | 0.003 | |
| Peak gradient (mmHg) | 76 ± 21 | 66 ± 19 | 0.049 | |
| Aortic valve area (cm2) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.922 | |
| PASP (mmHg) | 45 (32–53) | 54 (41–61) | 0.064 | |
| Aortic regurgitation >mild | 2 (3.4%) | 1 (4%) | 1 | |
| Mitral regurgitation >mild | 12 (20.7%) | 4 (16.0%) | 0.766 | |
| Tricuspid regurgitation >mild | 7 (12.0%) | 10 (40%) | 0.007 | |
CFS, clinical frailty score; LV EDD, left ventricular end diastolic diameter; LV ESD, left ventricular end systolic diameter; IVS, interventricular septal diameter; PWT, posterior wall thickness; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular planar systolic excursion; V max, trans-aortic peak velocity; PASP, pulmonary artery systolic pressure
Transcatheter aortic valve implantation details and complications
One patient in the conventional cohort died whilst waiting for a TAVI. All patients had transfemoral TAVI, except one in the conventional group who had a trans-caval TAVI. Procedural details and complications were similar between the two cohorts (Table 2). Two patients in the conventional cohort required intubation and ventilation- one because of procedure-related aortic dissection that required surgical intervention and the second because of pulmonary oedema during the TAVI.
Table 2 .
Procedural details and complications.
| Parameter | Conventional (n = 57)* | ASTRID-AS (n = 25) | P value | |
|---|---|---|---|---|
| Prosthesis type | Navitor | 4 (7.0%) | 2 (8.0%) | 0.904 |
| Portico | 18 (31.6%) | 6 (24.0%) | ||
| Sapien 3 | 26 (45.6%) | 11 (44.0%) | ||
| Sapien S3 ultra | 9 (15.8%) | 6 (24.0%) | ||
| Pre-dilatation | 26 (45.6%) | 8 (32.0%) | 0.249 | |
| Further valve intervention | 2 (3.5%) | 2 (8.0%) | 0.582 | |
| General anaesthesia | 2 (3.5%) | 0 (0.0%) | 1.000 | |
| Vasopressor/inotrope use | 4 (7.0%) | 1 (4.0%) | 1.000 | |
| Contrast administered (ml) | 213 (191–242) | 199 (167–221) | 0.058 | |
| Pacemaker | 4 (7.0%) | 3 (12.0%) | 0.670 | |
| Stroke | 0 | 0 | n/a | |
| Major vascular complication | 1 (1.8%) | 0 (0.0%) | 1.000 | |
| Minor vascular complication | 4 (7.0%) | 2 (8.0%) | 1.000 | |
| Major bleeding | 1 (1.8%) | 2 (8.0%) | 0.219 | |
| Minor bleeding | 4 (7.0%) | 2 (8.0%) | 1.000 | |
| Decrease in haemoglobin (g/dL) | 1.5 ± 1.3 | 1.3 ± 1.1 | 0.579 | |
| Red Cell transfusion | 5 (8.6%) | 2 (8.0%) | 1.000 | |
*One patient in the conventional cohort died in hospital before TAVI. Contrast administered is the total volume of contrast used from CT angiography and TAVI.
Hospital length of stay
Time to TAVI in the conventional vs. ASTRID-AS cohort was 22 (15–30) vs. 10 (6–12) days; P < 0.001, respectively. Length of stay was 24 (18–33) vs. 13 (8–18) days; P < 0.001, respectively (graphical abstract). On average, the ASTRID-AS pathway saved 13.4 bed days per patient. Table 3 identifies the duration of time in days for each part of the patients’ journey. Discharge to a destination other than the patient's home (another hospital, nursing care home, or rehabilitation centre) occurred in 4 (7.4%) patients in the conventional cohort and 2 (8.0%) patients in the ASTRID-AS cohort (P = 1.000).
Table 3 .
Duration of time in days for different parts of the patient journey according to each cohort.
| Parameter | Conventional (n = 58)* | ASTRID-AS (n = 25) | P value |
|---|---|---|---|
| Hospital admission to referral | 5 (3–12) | 2 (1–5) | <0.001 |
| Referral to BHC admission | 8 (5–12) | 3 (1–4) | <0.001 |
| TAVI to hospital discharge | 2 (1–4) | 2 (1–5) | 0.547 |
*Four patients in the conventional pathway died in hospital and were not included in the TAVI to hospital discharge analysis. BHC, Barts Heart Centre.
Mortality and morbidity
In the conventional cohort, one patient died pre-TAVI and three patients died within 30 days post-TAVI. The causes of mortality were cardiovascular for three patients and non-cardiovascular for one (supplementary results). There were no deaths at 30 days in the ASTRID-AS cohort. Eight patients in the conventional cohort and one patient in the ASTRID-AS cohort developed an AKI. The composite of death or AKI at 30 days occurred in 12 (20.7%) vs. 1 (4.0%) patients; P = 0.093, in the conventional vs. ASTRID-AS cohorts respectively (graphical abstract). In Cox regression analysis, ASTRID-AS was associated with a hazard ratio (HR) for death or AKI at 30 days of 0.206, 95% confidence interval (CI): 0.027–1.592; P = 0.130. Median time to TAVI for the study population was 15 days. After dividing the cohort, according to time to TAVI >15 days and ≤15 days, the former was associated with a hazards ratio of death or AKI at 30 days of HR: 2.345, 95% CI: 0.622–8.844; P = 0.208. Regression models and different stages of AKI are presented in the supplementary results.
Discussion
This is the first prospective study to address the impact of time to TAVI in patients with ADAS. There are three key findings from this study; first, shorter time to TAVI reduces hospital length of stay. Second, this can be achieved safely, without an increased risk of procedural complications. Lastly, shorter time to TAVI was associated with a trend towards less death or AKI at 30 days (graphical abstract).
ADAS is associated with significant economic, social, mortality and morbidity implications.1,3,9,10 The burden of ADAS is further augmented by its prevalence- up to 1 in 3 TAVI patients.15,16 The clinical implications of our findings provide an important step towards alleviating this burden. Reducing hospital length of stay has clear financial benefits and enables a greater number of patients to be treated with the same resources. Using a dedicated clinical pathway, 13.4 bed days were saved per patient- effectively doubling our capacity to treat ADAS patients compared with the conventional pathway. Our study has also demonstrated a signal indicating that the ASTRID-AS pathway may lower mortality (0 vs. 7%) and AKI (4 vs. 14%) compared with the conventional pathway. Although this pilot study is not powered to assess this outcome, our data provides the impetus for larger and longer studies.
Our findings lend support to previous studies that have identified the importance of quicker time to treatment in ADAS. Two studies have demonstrated increased mortality in ADAS patients with cardiogenic shock who had balloon aortic valvuloplasty >48 hours from presentation.17,18 Among patients without cardiogenic shock, two retrospective observational studies on ADAS patients found that delayed treatment with TAVI was associated with an increased mortality rate at 1 and 2 years.7,8 If shorter time to TAVI can improve outcomes, it is a simple strategy to implement with potentially a large impact. Akin to pathways involving revascularisation for myocardial infarction, where door to balloon time has prognostic implications,19 similar pathways may be beneficial for ADAS.
Historically, ADAS has been treated medically or with balloon aortic valvuloplasty with aortic valve replacement performed electively.3,20 Associated outcomes are worse with a strategy of first-line balloon aortic valvuloplasty compared with first-line TAVI.21,22 Our study goes a step further by not only supporting TAVI as a first-line therapy but also demonstrating that a dedicated clinical pathway for ADAS can provide quicker treatment, thereby reducing LoS and potentially improving clinical outcomes. Patients, especially the elderly, who have lengthy hospital admissions are susceptible to physical deconditioning, loose their independence, have reduced quality of life post-discharge and are likely to have social implications as well.6 Minimising hospital length of stay is of paramount importance for them.
Clinical pathways such as ASTRID-AS can utilise significant resources- organisational, echocardiography, CT department, and catheterisation lab time. Therefore, risk stratification of ADAS patients is important to identify which patients are at the highest risk of adverse events and therefore should get prioritised for expedited treatment. An echo-based classification has demonstrated prognostic utility in ADAS- patients with greater myocardial damage/dysfunction (echo stage > 2) have a higher risk of all-cause mortality at 1 year.23 Other predictors of adverse outcomes in the short and long-term need to be assessed to improve risk stratification.
Limitations
This was an open-label, non-randomised study and as such we cannot completely exclude differences in patient characteristics. Hospitals in each pathway were decided according to whether or not they were part of our NHS trust. This does create a selection bias. Despite these limitations the inclusion of hard endpoints, contemporary nature in a real-world setting and prospective design provide realistic evidence that can be replicated at other institutions. This study is not powered to determine changes in mortality and AKI. Larger studies will be required to assess clinical benefit. The definition of AKI was based on changes in blood tests obtained clinically during a patient's admission, rather than systematically at specific time points. Although all patients have almost daily blood tests whilst admitted, there may have been some episodes of AKI that may have been missed. We do not have details regarding the amount of intravenous fluids that were administered. We have assumed that this was similar between both cohorts, given that patients underwent similar procedures. If anything, patients in the ASTRID-AS cohort had a CT scan and TAVI procedure within a shorter time window, potentially increasing their contrast burden. 28% of patients referred for an urgent TAVI were excluded from this study as they did not meet the ASTRID-AS criteria. Our findings may not apply to ADAS patients with complex presentations and those who are very frail.
Conclusions
A dedicated clinical pathway for ADAS patients that shortens time to TAVI can reduce hospital length of stay without compromising patient safety and could potentially improve clinical outcomes.
Supplementary Material
Contributor Information
Kush P Patel, Institute of Cardiovascular science, University College London, London, EC1E 6BT, UK; Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Sumanto Mukhopadhyay, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Kerry Bedford, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Rhian Richards, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Helen Queenan, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Melanie Jerrum, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Judy Banton, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Mick Ozkor, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Anthony Mathur, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK; The William Harvey Research Institute, London, E1 4NS, UK.
Simon Kennon, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Andreas Baumbach, Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK; The William Harvey Research Institute, London, E1 4NS, UK; Yale University School of Medicine, New Haven, CT 06510. USA.
Michael J Mullen, Institute of Cardiovascular science, University College London, London, EC1E 6BT, UK; Barts Heart Centre, West Smithfield, London, EC1A 7BE, UK.
Funding
M.J.M. has received grants and personal fees from Edwards Lifesciences and personal fees from Abbott Vascular. K.P.P. is funded by a British Heart Foundation clinical research training fellowship grant (FS/19/48/34 523) and has an unrestricted research grant from Edwards Lifesciences that facilitated this work.
Disclosures
None apart from the above
Data availability
Data for this study are not available due to confidentiality reasons.
References
- 1. Kolte D, Khera S, Vemulapalli S, Dai D, Heo S, Goldsweig Aet al. . Outcomes following urgent/emergent transcatheter aortic valve replacement: insights from the STS/ACC TVT registry. JACC: Cardiovascular Interventions 2018;11:1175–1185. [DOI] [PubMed] [Google Scholar]
- 2. Wald DS, Williams S, Bangash F, Bestwick JP.. Watchful waiting in aortic stenosis: the problem of acute decompensation. Am J Med 2018;131:173–177. [DOI] [PubMed] [Google Scholar]
- 3. Patel K, Broyd C, Chehab O, Jerrum M, Queenan H, Bedford Ket al. . Transcatheter aortic valve implantation in acute decompensated aortic stenosis. Catheter Cardiovasc Interv 2020;96:E348–E354. Available from: 10.1002/ccd.28581 [DOI] [PubMed] [Google Scholar]
- 4. Shao R, Li J, Qu T, Fu X, Liao Y, Chen M.. Efficacy and safety of emergent transcatheter aortic valve implantation in patients with acute decompensated aortic stenosis: systematic review and meta-analysis. J Interv Cardiol 2021; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassan M, Tuckman HP, Patrick RH, Kountz DS, Kohn JL.. Hospital length of stay and probability of acquiring infection. Int J Pharm Healthc Mark 2010;4:324–338. Available from: 10.1108/17506121011095182 [DOI] [Google Scholar]
- 6. Smith TO, Sreekanta A, Walkeden S, Penhale B, Hanson S.. Interventions for reducing hospital-associated deconditioning: a systematic review and meta-analysis. Arch Gerontol Geriatr 2020;90:104176. Available from: https://www.sciencedirect.com/science/article/pii/S0167494320301709 [DOI] [PubMed] [Google Scholar]
- 7. Kaewkes D, Ochiai T, Flint N, Patel V, Patel J, Kim Iet al. . Transcatheter aortic valve implantation in patients with severe aortic stenosis hospitalized with acute heart failure. Am J Cardiol 2021;144:100–110. [DOI] [PubMed] [Google Scholar]
- 8. Lux A, Veenstra LF, Kats S, Dohmen W, Maessen JG, van ’t Hof AWJet al. . Urgent transcatheter aortic valve implantation in an all-comer population: a single-centre experience. BMC Cardiovascular Disorders 2021;21:550. Available from: 10.1186/s12872-021-02347-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell Iet al. . A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk Det al. . Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv [Internet] 2013;82:640–652. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ccd.24751 [DOI] [PubMed] [Google Scholar]
- 11. Scherner M, Wahlers T.. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis 2015 Sep;7:1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EHet al. . Updated standardized endpoint definitions for transcatheter aortic valve implantation : the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 13. Koifman E, Segev A, Fefer P, Barbash I, Sabbag A, Medvedovsky Det al. . Comparison of acute kidney injury classifications in patients undergoing transcatheter aortic valve implantation: Predictors and long-term outcomes. Catheter Cardiovasc Interv 2016;87:523–531. [DOI] [PubMed] [Google Scholar]
- 14. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot Pet al. . Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021;42:1825–1857. Available from: 10.1093/eurheartj/ehaa799 [DOI] [PubMed] [Google Scholar]
- 15. Popovic B, Molho A, Varlot J, Fay R, Metzdorf P, Elfarra Met al. . Prognostic influence of acute decompensated heart failure in patients planned for transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2020;96:E542–E551. [DOI] [PubMed] [Google Scholar]
- 16. Elbadawi A, Elgendy IY, Mentias A, Saad M, Mohamed AH, Choudhry MWet al. . Outcomes of urgent versus nonurgent transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2020;96:189–195. [DOI] [PubMed] [Google Scholar]
- 17. Debry N, Kone P, Vincent F, Lemesle G, Delhaye C, Schurtz Get al. . Urgent balloon aortic valvuloplasty in patients with cardiogenic shock related to severe aortic stenosis: Time matters. EuroIntervention 2018;14:e519–e525. [DOI] [PubMed] [Google Scholar]
- 18. Buchwald AB, Meyer T, Scholz K, Schorn B, Unterberg C.. Efficacy of balloon valvuloplasty in patients with critical aortic stenosis and cardiogenic shock - The role of shock duration. Clin Cardiol 2001;24:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park J, Choi KH, Lee JM, Kim HK, Hwang D, Rhee Tet al. . Prognostic implications of door-to-balloon time and onset-to-door time on mortality in patients with ST-Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J Am Heart Assoc 2019;8:e012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdelaziz M, Khogali S, Cotton JM, Meralgia A, Matuszewski M, Luckraz H.. Transcatheter aortic valve implantation in decompensated aortic stenosis within the same hospital admission: early clinical experience. Open Hear [Internet] 2018; 5. Available from: https://openheart.bmj.com/content/5/2/e000827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bongiovanni D, Kühl C, Bleiziffer S, Stecher L, Poch F, Greif Met al. . Emergency treatment of decompensated aortic stenosis. Heart 2018;104:23–29. [DOI] [PubMed] [Google Scholar]
- 22. Ali N, Patel P, Wahab A, Das A, Blackman DJ, Cunnington MSet al. . A cohort study examining urgent and emergency treatment for decompensated severe aortic stenosis. J Cardiovasc Med [Internet] 2021;22. Available from: https://journals.lww.com/jcardiovascularmedicine/Fulltext/2021/02000/A_cohort_study_examining_urgent_and_emergency.9.aspx [DOI] [PubMed] [Google Scholar]
- 23. Patel KP, Badiani S, Ganeshalingam A, Vijayakumar M, Thornton G, Mathur Aet al. . Preprocedural prognostic factors in acute decompensated aortic stenosis. Am J Cardiol 174,96–100, 2022; Available from: 10.1016/j.amjcard.2022.03.037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study are not available due to confidentiality reasons.