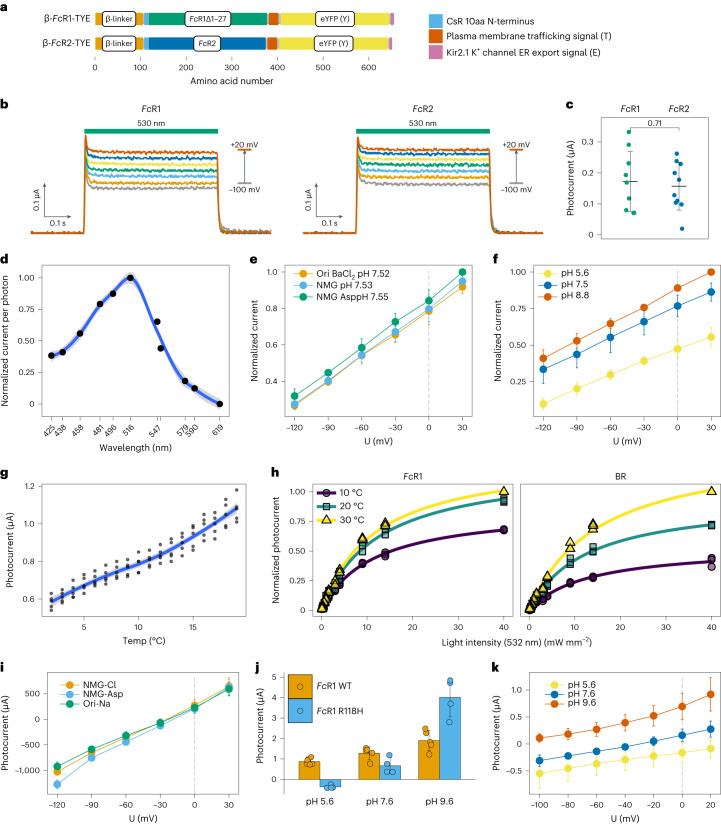
Fig. 4. Biophysical characterization of F. cylindrus rhodopsin variants (FcR1/2) as cold-adapted green light-driven proton pumps.
a, Schematics of DNA constructs used for TEVC analysis in X. laevis oocytes. Orange, amino acids 1–105 of rat gastric H+/K+-ATPase β-subunit fragment (β-linker); sky blue, amino acids 1–10 of C. subellipsoidea rhodopsin (CsR); bluish green, N-terminally truncated FcR1 (FcR1Δ27); blue, full-length FcR2; vermillion, the plasma membrane trafficking signal from Kir2.1 K+ channel (T); yellow, enhanced yellow fluorescent protein (eYFP); reddish purple, the endoplasmic reticulum export signal from Kir2.1 K+ channel (E); grey, FLAG-tag (FLAG). b, Voltage dependence of the green light-induced pump signal of FcR1 and FcR2. Currents were recorded at incremental membrane potential steps of 20 mV from −100 mV (grey) to +20 mV (vermillion). A 530 nm laser was used for illumination. c, Comparison of FcR1 and FcR2 photocurrents. Statistics are displayed as mean ± s.d. (n = 10) and the P value displayed was determined by a two-sided unpaired t-test. d, Action spectrum of FcR1. FcR1 photocurrent was measured in NMG buffer with pH 7.5 at a membrane potential of −20 mV. The photocurrent was normalized to the FcR1 photocurrent with 516 nm light illumination and is shown as arbitrary units. Statistics are displayed as mean ± s.d. (n = 3) and the blue line shows smoothed conditional means (loess smooth) of photocurrents, including their 0.95 confidence intervals (grey). e, FcR1 photocurrents in Ori BaCl2 pH 7.5, NMG pH 7.5 and NMG Asp pH 7.55 buffers and at membrane potentials from −120 mV to +30 mV. The photocurrent was normalized to the FcR1 photocurrent in NMG Asp pH 7.55 buffer at a membrane potential of +30 mV and is shown as arbitrary units. A 532 nm laser was used for illumination. Statistics are displayed as mean ± s.d. (n = 4). f, FcR1 photocurrent measured in NMG buffer with pH 5.6, 7.5 and 8.8 at membrane potentials from −120 mV to +30 mV. The photocurrent was normalized to the FcR1 photocurrent in NMG pH 8.8 buffer at a membrane potential of +30 mV and is shown as arbitrary units. A 532 nm laser was used for illumination. Statistics are displayed as mean ± s.d. (n = 3). g, FcR1 photocurrents at different temperatures (n = 5). Oocytes were incubated in ND96 buffer containing 1 µM all-trans-retinal, and 532 nm green light was used for illumination. The blue line shows smoothed conditional means (loess smooth) of photocurrents, including their 0.95 confidence intervals (grey). h, Comparison of FcR1 and BR photocurrents at different temperatures and light intensities. Photocurrents were tested at 10°C (ripe plum, circles), 20°C (bluish green, squares) and 30°C (light yellow, triangles) and different light intensities (n = 3). Oocytes were incubated in ND96 buffer containing 1 µM all-trans-retinal and 532 nm green light was used for illumination. Photocurrents were normalized and are shown as arbitrary units. i, Green-light-induced photocurrents of FcR1 R118H mutant in three different extracellular buffers: NMG Asp pH 7.7, NMG pH 7.6 and Ori BaCl2 pH 7.6. Currents were recorded at incremental membrane potential steps of 30 mV from −120 mV to −30 mV. Statistics are displayed as mean ± s.d. (n = 5). j, Comparison of stationary photocurrents of FcR1 wild type (WT, n = 5) and FcR1 R118H (n = 4) in NMG buffers of different pHs at a membrane potential of 0 mV. Statistics are displayed as mean ± s.d. k, Green-light-induced photocurrents of FcR1 R118H mutant in NMG buffers of different pHs. Currents were recorded at incremental membrane potential steps of 20 mV from −100 mV to +20 mV. Statistics are displayed as mean ± s.d. (n = 5).