Abstract
The goal of the current study was to enhance the measurement of the pediatric chronic pain experience through a methodologically rigorous approach. This paper outlines the development and initial validation of a pain intensity measure for pediatric patients with chronic pain using PROMIS® methodology. Measure development incorporated feedback from children with painful conditions. Based on input from pediatric participants and content experts, four candidate items assessing pain intensity were included for large scale testing. Children completed self-report items pertaining to their pain experience that were developed as part of a larger pool of new candidate PROMIS® pediatric pain domain items as well as measures of pain interference, depressive symptoms, fatigue, pain behavior, pain intensity, and pain catastrophizing. The final sample for the large scale testing included N = 442 pediatric patients between the ages 8 to 18 years (Mean age = 13.54, SD = 2.78; 71.27% female) experiencing chronic pain. Psychometric analysis resulted in a final measure that included three items with evidence of reliability (Cronbach alpha = 0.82) and convergent validity. The Likert format of the response options may be preferable to the traditional numeric rating scale for use in pediatric populations who experience chronic pain based on patients’ feedback, which was directly utilized in designing the scale. Further, the inclusion of fewer and clinically meaningful response options should reduce ambiguity for young respondents.
Keywords: Pediatric pain, item response theory, patient reported outcomes, chronic pain, pain measurement
Perspective:
We have developed and evaluated a clinically sensitive and psychometrically precise 3-item pain intensity measure with Likert-type responses for self-report use among children and adolescents ages 8–18 with chronic pain. Development of the item content and response options included input from children and adolescents with chronic pain. The development of pain intensity items with pediatric appropriate language, and labeled, fewer response options to yield maximal clinically meaningful information improves the precision of pain intensity measurement in children.
Introduction
Appropriate management of pediatric pain relies on accurate assessment of pain intensity. The gold standard for assessing pain intensity is by self-report: “pain is what the patient says it is, and occurs when he or she says it does”27. A number of patient-reported pain intensity measures are widely used in pediatric populations, including visual analog scales (VAS26), numeric rating scales (NRS28,32), and faces pain scales (FPS-R2,18); however the psychometric precision of these measures are limited3. The PedIMMPACT consensus statement has provided guidelines for selection of measures for different age groups of children, recommending the FPS-R for children aged 4–12 and the VAS in children ages 8 and above29. The NRS is widely used and extensively studied in adults, and recent studies have found evidence of reliability and validity in children7. Despite wide clinical utilization of these measures, the evidence for their measurement properties is surprisingly weak in pediatric chronic pain as described in a recent comprehensive review3. Additionally, none of these measures, with exception of the FPS-R, was developed with input from pediatric patients on question wording, content, and response options. Thus, they rely on an untested assumption that children will assign similar meanings to convey different ratings, and will interpret and respond to these questions about their pain the same way adults do. Indeed, a recent review7 identifies issues with the administration of the NRS in children, including a lack of consensus about labeling the anchors and instructions. These issues could be mitigated by including child and adolescent feedback on wording and response options.
Evidence in the general measurement literature finds that children prefer Likert-type response scales over numeric or visual analog scales33 and that a scale with many options, such as the NRS, may exceed pediatric respondents’ capacity to discriminate, contributing to measurement error1. Specifically, seven or more response options has been associated with a decrease in measure reliability6. Research in measurement scaling also indicates that use of verbal labels on response options is superior in terms of precision, quality, and reliability compared to only providing labels on anchor options1,5. Notably, no studies have used modern test theory methods to develop or validate commonly used measures of pain intensity in pediatrics. The PROMIS pediatric measure presented in the current paper offers labeled Likert-type response options, developed with input from children with chronic pain and rigorous psychometric methods to provide an enhanced understanding of pain intensity.
The goal of the current study was to develop and test a brief self-report measure with short response sets to better estimate the intensity of pain in pediatric patients with chronic pain that accurately reflects their pain experiences. The current work was part of a larger project to develop pediatric self-report measures of the different dimensions of pain using PROMIS® methodology. The National Institutes of Health launched the Patient-Reported Outcomes Measurement Information System® (PROMIS) initiative9 in 2005 to develop new patient-reported outcome (PRO) measures including measures of pain quality using a mixed methods approach, incorporating qualitative research14,21 and modern measurement theory31. The overarching goal was to improve the reliability, precision, responsiveness, validity, and efficiency of patient-reported outcomes (PROs) assessment in adult- and child-reported measurement of health. In the current investigation, we outline the development and validation of the pediatric pain intensity measure using PROMIS® methodology and drawing on the extant measurement literature on scaling and anchoring of item responses for pediatric populations. Specifically, we sought to improve current methods of assessing pediatric chronic pain by developing a pain intensity measure by and for pediatric populations, with evidence of good model fit based on confirmatory factor analysis and evidence of convergent validity.
Methods
Participants
The intent of our study design was to test if new PROMIS® pain intensity items would be suitable for measurement across chronic/recurrent painful pediatric conditions. We recruited pediatric patients (N=448) between the ages 8 to 18 years with a diagnosis of a painful chronic condition, including juvenile idiopathic arthritis (JIA), non-inflammatory chronic pain, such as juvenile fibromyalgia (JFM), and sickle cell disease (SCD). Patients were recruited through outpatient clinics (e.g., Pain Management Clinic, Rheumatology Clinic) at three pediatric medical centers in three regions of the United States (Midwest, n = 230; Northeast, n = 138; and Southeast, n = 80). Patients with concurrent medical conditions (e.g., diagnosis of chronic disease conditions other than the primary painful condition), psychiatric conditions that could in themselves result in decreased health related quality of life, or cognitive impairment (e.g. autism, developmental delay) that would preclude completion of self-report items, were excluded. Study coordinators identified eligibility criteria based on review of electronic medical records. During recruitment, candidates were screened for whether they had experienced pain in the past 7 days to ensure that those with recurrent pain conditions (JIA and SCD) were currently or within a few days of experiencing an episode of pain because the recall period of PROMIS measures is typically 7 days. Participants were limited to those who could read and speak English. This study was approved by the Institutional Review Boards at each institution and patients and parents provided written informed consent/assent for participation in the study. Notably, n = 6 patients did not complete the candidate PROMIS® pediatric pain intensity items and were excluded from analyses, resulting in a final sample size of N = 442 included in the current study.
Procedure
Patients who met all study inclusion criteria at the time of their medical appointment, based on the medical chart review, were approached by a clinical research coordinator in the clinic and invited to participate in the study. Children completed self-report items pertaining to their pain experience that were developed as part of a larger pool of new candidate PROMIS® pediatric pain domain items16,23,24. In addition, legacy self-report measures of pain interference, depressive symptoms, fatigue, pain behavior, pain intensity (NRS-11), and pain catastrophizing were also collected. Caregivers provided data on socio-demographic information and health history. All assessments were completed in person in an outpatient medical setting, sociodemographic forms using paper and pencil and test items were administered on laptop computer. Procedures were standardized across three study sites.
Development & Revision of Pain Intensity Items
PROMIS® researchers have conceptualized pain experiences into three sub-domains: Pain behavior, pain interference, and pain quality. Pain intensity, which is an aspect of pain quality, refers to “how much a person hurts”. Candidate items for the pediatric pain intensity measure were developed following the rigorous, methodological standards of PROMIS®8,14. The methodology and results of this iterative qualitative process are comprehensively detailed in Farrell, Kashikar-Zuck, Jacobson, Correia, Dampier, Verkamp, Segerman, and Morgan16, Jacobson, Farrell, Kashikar-Zuck, Seid, Verkamp and Morgan23, and Jacobson, Kashikar-Zuck, Farrell, Barnett, Goldschneider, Dampier, Cunningham, Crosby, and Morgan24, but summarized here. Items were developed or revised based on a literature review, expert feedback, cognitive interviews, focus groups, and semi-structured interviews conducted with N = 34 youth ages 8–18 with a diagnosis of JIA or chronic pain condition such as migraines, chronic headaches, abdominal pain, and musculoskeletal pain (81% female, mean age of 13.8; for full demographic detail on this subsample, see Jacobson, Farrell, Kashikar-Zuck, Seid, Verkamp and Morgan23). Eligible patients were introduced to the study by their primary treating physician and those who were interested were contacted by the research coordinator. Once the initial pool of items was generated from a literature review, content experts in the field of pain assessed and revised the items as needed for clarity of concept and language, and to adhere to PROMIS® formatting. A translation expert reviewed item wording to evaluate potential for translatability into other languages and cross-cultural validation by identifying conceptual or linguistic ambiguities. Interviewers asked participants about the subjective meaning of the domain, each item, and about the participants’ own experiences (e.g., “What kinds of things come to mind when I say the word______?”). Participants also completed the candidate items for the measure and were asked about comprehension and preferred word choice. Participants discussed difficulties they had completing the items, what they believe the item means, and how they choose their responses. The goal was that the items and response options were written in a way that was mutually understood by patients and the research team. Items were modified or excluded based on the participants’ feedback. For example, some participants indicated difficulty understanding the term “intense” in the original pain intensity item (e.g., “how intense is your average pain”), and thus this term was modified to “bad”. There was also mixed feedback on the best word choice for the item intended to assess the typical pain intensity experienced over the past 7 days, with the choice of wording between “usual” versus “average”. Thus, the study team decided to include both items in our large scale quantitative testing for evaluation and comparison of each item’s psychometric qualities.
Based on the qualitative input from pediatric participants and our content experts, four candidate items assessing pain intensity were included for large scale testing: “In the last 7 days, how bad was your pain at its worst?”, “In the last 7 days, how bad was your usual pain?”, “In the past 7 days, how bad was your average pain?”, .and “What is your level of pain right now?”. Response options for all items were: “0 = Had no pain”, “1 = Mild”, “2 = Moderate (medium amount)”, “3 = Severe”, “4 = Very severe”. Recommended scoring for PROMIS measures follows standard expected a posteriori (EAP) scoring for response patterns4. However, to enhance usability of PROMIS measures, acceptable T-score estimates can be obtained from the summed scores using look-up tables.
Measures
PROMIS® Pediatric Measures35.
Participants completed measures of: 1) pain interference (8 item short form v1.0)34 i.e., difficulties in completing daily activities, socioemotional problems, and impairment in physical functioning due to pain, 2) depressive symptoms (8 item short form v1.0)22, 3) fatigue (10 item short form v1.0)25, and pain behavior (8 item short form v1.0)13, i.e., behaviors that typically communicate to others that an individual is experiencing pain that include observable displays of pain and verbal reports of pain. These PROMIS® measures assessed presence of symptoms in the past 7 days via a 5-point Likert-type scale. Response options were “0 = never”, “1=almost never”, “2=sometimes”, “3=often”, and “4=almost always”. The pain behavior measure also contained a “had no pain” response option. Higher scores indicate greater symptoms or a higher level of the construct being measured. Pediatric short forms were used because these measures have been found to be more precise compared to adaptive PROMIS® measures in children8. T-scores were calculated for all measures.
Pain Intensity Numeric Rating Scale (NRS).
Overall pain intensity in the past week was collected via patient self-report using a four-item 0 to 10 numeric rating scale (NRS) based on the Brief Pain Inventory10. Items asked about the worst pain in the last week, average pain in the last week, pain at its least in the last week, and pain right now. Mean scores were calculated for the 4 items.
Pain Catastrophizing.
The Pain Catastrophizing Scale - Child Version (PCS-C)11, contains 13 Likert-type items assessing negative attitudes towards the pain, such as rumination, magnification and feelings of helplessness. Response options include: not at all (0), mildly (1), moderately (2), severely (3), and extremely (4). Total scores range from 0 to 52, with higher scores reflecting greater pain catastrophizing.
Statistical Analyses
Construct Validity.
Confirmatory factor analysis (CFA) and item response theory (IRT) methods were conducted to test the psychometric properties of the four candidate PROMIS® pediatric pain intensity items using Mplus version 8.130 with a mean-and-variance adjusted weighted least squares estimator. Model fit was examined using empirically validated fit indices and levels suggested by Hu and Bentler19. Specifically, a root mean square error of approximation (RMSEA) < .05, and a comparative fit index (CFI) and Tucker-Lewis Index (TLI) > 0.95 indicate the hypothesized unidimensional model structure fit the data well.
Reliability.
To determine test information and coverage across the target construct, item and test information curves and the range of threshold parameters were evaluated. Note that information curves are a methodological advance over traditional methods of assessing reliability of a measure and indicate the precision of an item or measure along the underlying construct continuum. Unlike classical test theory approaches which assume that information is constant across all levels of the underlying construct and provides one value (such as a coefficient alpha), information derived from item response theory (IRT)-based models varies by trait level. We also estimated internal consistency (Cronbach’s alpha) of the final set of items.
Convergent Validity.
To assess the convergent validity of the PROMIS® pediatric pain intensity measure, we examined bivariate correlations with the PROMIS® pediatric pain interference short form, pain behavior short form, depressive symptoms short form, fatigue short form, as well as scores on the pain catastrophizing scale and the pain intensity NRS scale.
Results
Descriptive Statistics
A total of 442 children (Mean age = 13.54, SD = 2.78) participated in the study. 63.49% identified as Caucasian and 30.39% identified as African-American. 4.1% identified themselves as Hispanic. Consistent with the higher prevalence of chronic pain including JFM in girls, particularly in adolescents35, and female predominance in JIA, we observed a larger proportion of female participants (71.27%) in our sample. In total, there were 175 participants with JIA, 115 participants with SCD, and 151 participants with JFM (N = 1 was missing).
Construct Validity and Reliability
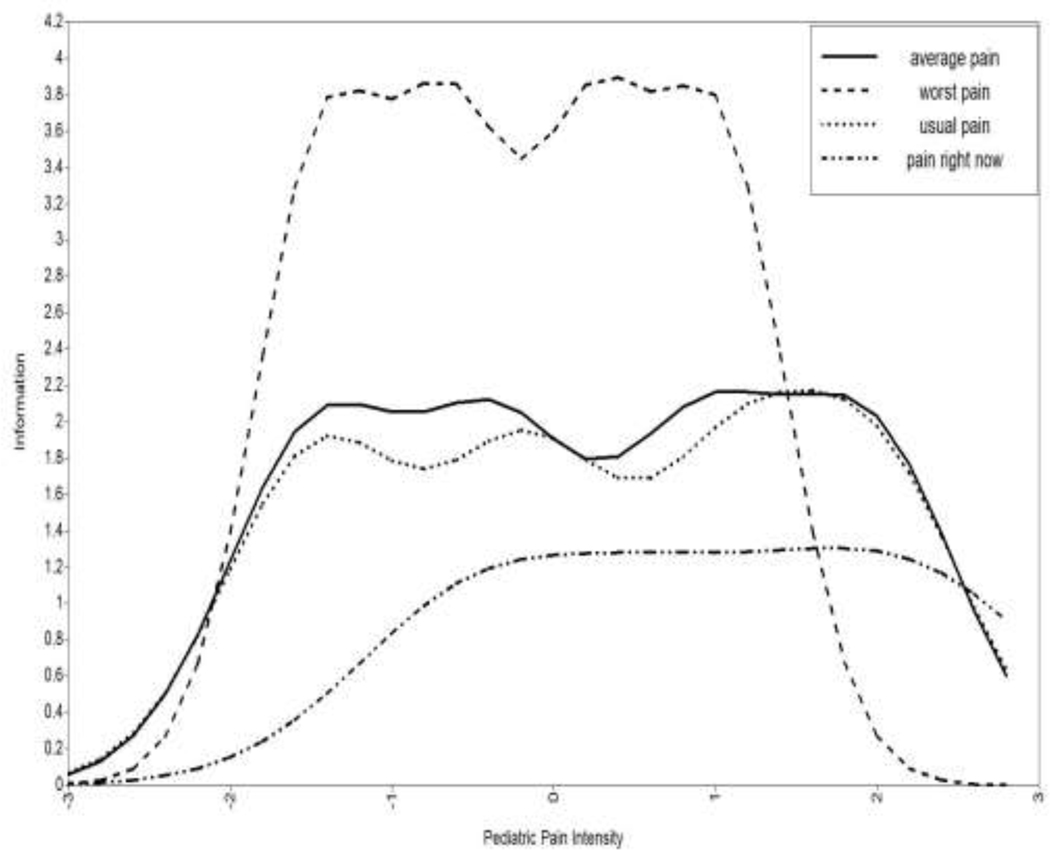
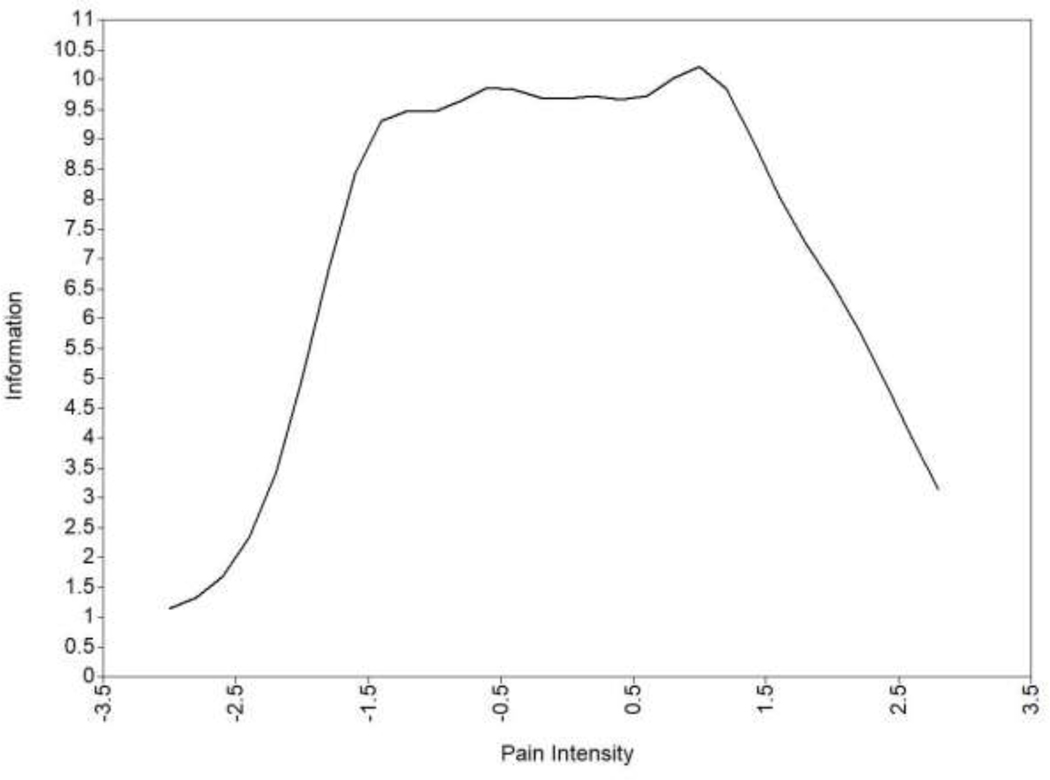
The CFA for the four candidate PROMIS® pediatric pain intensity items demonstrated good fit to a unidimensional model (χ2 (2) = 1.51, p = 0.47, RMSEA = 0, TLI = 1.00, CFI = 1.00). The factor loadings (which represent how strongly each item is related to the underlying trait; sometimes referred to as “slope”) ranged from 1.14 to 2.09 and were statistically significant, indicating that each item was strongly related to the underlying construct (Table 1). Location threshold is the level of the underlying construct (e.g., pain intensity) where a participant is more likely than not to choose the observed response option in the higher category than the one adjacent to it. For example, for the first item, location threshold 4 = 2.97 (the boundary between an observed response of 3 versus a 4) indicates that an individual is more likely to choose the “4= very severe” response option versus the “3 = severe” response option when their underlying pain intensity is at least 2.97 standard deviations above the mean. The location thresholds observed in our data indicated broad coverage across the pain intensity construct. Item information curvesa (i.e., estimates of measurement precision/reliability; see Figure 1) indicated that 2 items overlapped almost completely in terms of information. Specifically, “how intense was your average pain?” and “how bad was your usual pain?” provided very similar information. Thus, we considered them redundant and excluded the item “how intense was your average pain?” given that children who participated in the qualitative phase of the study tended to prefer the phrasing “usual”. This resulted in a final measure that included a total score of three items. Factor loadings and thresholds from the model including only these three final items are presented in Table 2. Information was highest for the item “how bad was your pain at its worst?” and lowest for the item “What is your level of pain right now?” Overall information for the three items was high between −2 and +2 standard deviations around the mean (see Figure 2), and Cronbach’s alpha for the final 3 items of the PROMIS pediatric pain intensity measure was 0.82. PROMIS measures are all scored on the T-score metric, in which 50 is the mean of a relevant reference population, and 10 is the standard deviation of that population. Across all PROMIS measures, higher scores indicates more of the concept being measured. Specific to pain intensity, a higher score means higher pain intensity. See Appendix A for the final measure and Appendix B for the raw score to T-score look-up table. More information about scoring and interpreting measures can be found at http://www.healthmeasures.net/score-and-interpret/interpret-scores.
Table 1.
Item Parameters for Candidate PROMIS® Pediatric Pain Intensity Items.
| Item | Factor Loading | Location Threshold 1 | Location Threshold 2 | Location Threshold 3 | Location Threshold 4 |
|---|---|---|---|---|---|
| In the past 7 days, how intense was your average pain? | 1.56 | −2.24 | −0.57 | 1.45 | 2.97 |
| In the past 7 days, how bad was your pain at its worst? | 2.09 | −2.95 | −1.33 | 0.53 | 2.12 |
| In the past 7 days, how bad was your usual pain? | 1.51 | −2.17 | −0.25 | 1.76 | 2.90 |
| What is your level of pain right now? | 1.14 | −0.50 | 0.61 | 1.77 | 2.65 |
Figure 1.
Item Information Curves for Candidate PROMIS® Pediatric Pain Intensity Items
average pain = In the past 7 days, how intense was your average pain?
worst pain = In the past 7 days, how bad was your pain at its worst?
usual pain = In the past 7 days, how bad was your usual pain?
pain right now = What is your level of pain right now?
Table 2.
Item Parameters for Final PROMIS® Pediatric Pain Intensity Items.
| Item | Factor Loading | Location Threshold 1 | Location Threshold 2 | Location Threshold 3 | Location Threshold 4 |
|---|---|---|---|---|---|
| In the past 7 days, how bad was your pain at its worst? | 2.24 | −3.12 | −1.41 | 0.56 | 2.23 |
| In the past 7 days, how bad was your usual pain? | 1.48 | −2.14 | −0.24 | 1.74 | 2.86 |
| What is your level of pain right now? | 1.11 | −0.49 | 0.60 | 1.74 | 2.60 |
Figure 2.
Total Information for the Final PROMIS® Pediatric Pain Intensity Measure
Note: Information curves are analogous to reliability of measurement and indicate the precision of an item or measure along the underlying construct continuum, which varies across different levels of the trait. Information = 10 is comparable to internal consistency reliability of approximately .90; information = 5 is comparable to internal consistency reliability of approximately .80.
Convergent Validity
We observed strong correlations between the T-scores on the PROMIS® pediatric pain intensity measure and the NRS, r = 0.85, p < .001. We also observed moderate to strong correlations between scores on the PROMIS® pediatric pain intensity measure and scores on the PROMIS® pediatric pain interference short form, r = .67, p < .001, PROMIS® pediatric pain behavior short form, r = .60, p < .001, PROMIS® pediatric fatigue short form, r = 58, p < .001, PROMIS pediatric depressive symptoms, r = 0.42, p < .001, and the pain catastrophizing scale, r = 0.58, p < .001.
Discussion
This paper describes the development and psychometric validation of a new self-reported pain intensity measure for children and adolescents with chronic pain as part of the PROMIS® initiative. We developed a more linguistically targeted measure of pediatric pain intensity for patients 8–18 years of age using state of the art measurement science. By directly using the language and word choice of pediatric patients who were experiencing recurrent or chronic pain, the items can be more accurately understood in the manner intended by children. The format of the response options with five labeled, Likert style responses appears to be the preferred choice compared to the traditional 11-point numeric scale due to interpretability of the scale and fewer, more meaningful options to minimize ambiguity in the response options. Large scale psychometric testing among children with chronic painful conditions supported the reliability of the measure and convergent validity with other self-reported measures. Specifically, we observed a high correlation with the pain intensity NRS, and strong to moderate correlations with expected similar constructs (pain interference, pain behavior, depressive mood, fatigue, and pain catastrophizing) were also observed.
In health care, 0–10 numeric rating scales (NRS) of pain intensity are customarily used in routine clinical care and research studies. However, in practice, survey respondents discriminate between fewer gradations on these kinds of self-report measures, and children tend to discriminate between even fewer categories than adults. When specifically evaluating the response options on extant pain intensity measures, Edelen and Saliba15, using item response theory methods, showed that among adults, the 11 categories of the NRS do not yield unique information and have low discrimination, unlike verbal descriptive scales (VDS). Specifically, response categories 4, 6, and 9 cover no unique area of the underlying trait measured. Indeed, all categories are overlapping to large degree with neighboring categories, suggesting that someone who chooses a 4 is indistinguishable from someone who chooses a 3 or a 5. Thus, there are limitations to using the current NRS system to provide a nuanced understanding of the pediatric pain experience. Research in measurement scaling also indicates that use of verbal labels on response options (versus numeric ratings) is superior in terms of precision, quality, and reliability. For example, Borgers, Hox, and Sikkel5 found that providing children (ages 8–16) fully labeled, specific response options produces the best quality data in pediatric self-report research; and Beckstead1 found that adding verbal labels to all numerically labeled response options for children improves reliability of the overall measure. The PROMIS pediatric pain intensity measure allows for a more methodologically rigorous and clinically meaningful approach to understanding pediatric chronic pain. The Likert-type response options offered by our measure, developed with input from children with chronic pain, provides an enhanced understanding of the patients’ experience of their own pain intensity.
The final 3-item PROMIS pediatric pain intensity measure makes available a new, brief, psychometrically precise measure for use in research and clinical settings. The 3 items consider the intensity of pain “right now”, the intensity level of “worst” pain, and “usual” pain in the past 7 days. Our empirical data demonstrated that of the 3 items, “what is your level of pain right now” was the least informative item of those tested with respect to differentiating between patients with chronic pain. While “Level of pain right now” may be useful in an acute care or post-operative setting, this study underscores that for understanding chronic pain severity, the “pain right now” item appears to be less informative, and alone it is insufficient to characterize pain intensity in chronic conditions17. The most informative item in our study to differentiate the intensity of pain experienced by patients with chronic painful conditions is the highest level of pain experienced in the past week. Some approaches to understanding chronic pain, such as the chronic pain grading system, incorporate both highest and average pain into a single measure given the added clinical utility of assessing the highest pain rating20,36,37. This method has been applied in pediatric chronic pain populations with clinically meaningful results12, though prior research in this area has been limited to use of NRS pain ratings. Given the psychometric findings in the current research, we recommend that researchers and clinicians use the global score from all 3 items, as it is a more reliable index of pediatric pain intensity than using only a single item. The total raw score for this measure is simply the sum of the values of the response to each of the three questions. Then, the score conversion table provided in Appendix B translates the total raw score into a standardized T-score. This combined measure of pain intensity can be used in future research and clinical settings to provide an easy to administer, clearly understandable and robust indicator of pain intensity for children and adolescents with chronic pain. Future research on clinically meaningful endpoints and sensitivity to change is warranted.
Limitations and Directions for Future Research
This study was limited to United States participants and only included patients with three types of chronic pain conditions. Further validation of this measure with a more diverse sample with respect to including youth with a variety of acute, recurrent and chronic pain conditions would support generalizability of its use. Cross-cultural validation and translation into other languages is also needed for broader use. Additional psychometric properties, such as test re-test reliability, should be investigated in future studies. Further research is needed to understand minimally clinically important difference in this measure as well as understanding severity thresholds that may aid in clinical utility of the measure. Additionally, linking PROMIS pediatric pain intensity scores with existing legacy pain intensity measures will aid in interpretation of the PROMIS measures and link this measure with frequently-used benchmarks of severity on this construct.
Finally, this new measure asks patients to recall pain in the last 7 days. This may be problematic for recurrent pain that only occurs once, twice or three times a month (such as migraine or chronic abdominal pain). Indeed, to understand less frequent acute or recurrent pain, daily diaries or an event-based approaches might be more appropriate. However, we believe this approach offers a timely and important direction for understanding pediatric chronic pain, which will inform future research.
Conclusion
We have developed and psychometrically evaluated a clinically sensitive and psychometrically precise 3-item pain intensity measure with Likert-type responses for self-report use among children and adolescents ages 8–18 (see Appendix A for the final measure). Development of the item content and response options included input from children and adolescents with chronic pain. The resulting measure shows good reliability and validity and is recommended for use in research and clinical care with pediatric populations. The development of pain intensity items with pediatric appropriate language, and labeled, fewer response options to yield maximal clinically meaningful information improves the precision of pain intensity measurement in children.
Supplementary Material
Acknowledgements
We are indebted to C. Jeffrey Jacobson, PhD for his extensive contributions to the qualitative work that underlay development of the PROMIS® pediatric pain intensity measure. This project would not have been possible without the excellent research assistance from Jenna Tress, Leann Schilling, and Caravella McCuistian for assistance with patient recruiting, administration of questionnaires and regulatory compliance. We would also like to acknowledge Melissa Daeschner for her assistance in preparing this manuscript. Finally, we are very grateful to the patients and parent participants in this research study.
Funding:
The Patient-Reported Outcomes Measurement Information System® (PROMIS) is an NIH Roadmap initiative to develop a computerized system measuring PROs in respondents with a wide range of chronic diseases and demographic characteristics. PROMIS II was funded by cooperative agreements with a Statistical Center (Northwestern University, PI: David Cella, PhD, 1U54AR057951), a Technology Center (Northwestern University, PI: Richard C. Gershon, PhD, 1U54AR057943), a Network Center (American Institutes for Research, PI: Susan (San) D. Keller, PhD, 1U54AR057926) and thirteen Primary Research Sites which may include more than one institution (State University of New York, Stony Brook, PIs: Joan E. Broderick, PhD and Arthur A. Stone, PhD, 1U01AR057948; University of Washington, Seattle, PIs: Heidi M. Crane, MD, MPH, Paul K. Crane, MD, MPH, and Donald L. Patrick, PhD, 1U01AR057954; University of Washington, Seattle, PIs: Dagmar Amtmann, PhD and Karon Cook, PhD, 1U01AR052171; University of North Carolina, Chapel Hill, PI: Darren A. DeWalt, MD, MPH, 2U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, MD, PhD, 1U01AR057956; Stanford University, PI: James F. Fries, MD, 2U01AR052158; Boston University, PIs: Stephen M. Haley, PhD and David Scott Tulsky, PhD (University of Michigan, Ann Arbor), 1U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, MD and Brennan Spiegel, MD, MSHS, 1U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, 2U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, PhD (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, PhD, U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, MD, MSCE, 17 1U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, MD, 1U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, 2U01AR052186). NIH Science Officers on this project have included Deborah Ader, PhD, Vanessa Ameen, MD, Susan Czajkowski, PhD, Basil Eldadah, MD, PhD, Lawrence Fine, MD, DrPH, Lawrence Fox, MD, PhD, Lynne Haverkos, MD, MPH, Thomas Hilton, PhD, Laura Lee Johnson, PhD, Michael Kozak, PhD, Peter Lyster, PhD, Donald Mattison, MD, Claudia Moy, PhD, Louis Quatrano, PhD, Bryce Reeve, PhD, William Riley, PhD, Ashley Wilder Smith, PhD, MPH, Susana Serrate-Sztein, MD, Ellen Werner, PhD and James Witter, MD, PhD.
Footnotes
Note that information curves are a function of the model parameters, i.e., slopes and thresholds.
Disclosures: No conflicts of interest exist for any of the study authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beckstead JW: On measurements and their quality. Paper 4: verbal anchors and the number of response options in rating scales. Int J Nurs Stud 51(5): 807–814, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB: The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 41(2): 139–150, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Birnie KA, Hundert AS, Lalloo C, Nguyen C, Stinson JN: Recommendations for selection of self-report pain intensity measures in children and adolescents: a systematic review and quality assessment of measurement properties. Pain 160(1): 5–18, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Bock RD, Mislevy RJ: Adaptive EAP estimation of ability in a microcomputer environment. Appl Psych Meas 6(4): 431–444, 1982. [Google Scholar]
- 5.Borgers N, Hox J, Sikkel D: Response quality in survey research with children and adolescents: The effect of labeled response options and vague quantifiers. Int J Public Opin Res 15(1): 83–94, 2003. [Google Scholar]
- 6.Borgers N, Sikkel D, Hox J: Response effects in surveys on children and adolescents: The effect of number of response options, negative wording, and neutral mid-point. Qual Quant 38(1): 17–33, 2004. [Google Scholar]
- 7.Castarlenas E, Jensen MP, von Baeyer CL, Miro J: Psychometric properties of the Numerical Rating Scale to assess self-reported pain intensity in children and adolescents: A systematic review. Clin J Pain 33(4): 376–383, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Cella D, Chang CH: A discussion of item response theory and its applications in health status assessment. Med Care 38(9): 66–72, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, on behalf of the PROMIS Cooperative Group: The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63(11): 1179–1194, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM: Pain assesment: Global use of the Brief Pain Inventory. Annal Aca Med 23: 129–138, 1994. [PubMed] [Google Scholar]
- 11.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K: The child version of the Pain Catastrophizing Scale (PCS-C): A preliminary validation. Pain 104(3): 639–646, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham NR, Jagpal A, Peugh J, Farrell MK, Cohen MB, Mezoff AG, Lynch-Jordan A, Kashikar-Zuck S: Risk categorization predicts disability in pain-associated functional gastrointestinal disorders after 6 months. J Pediatr Gastroenterol Nutr 64(5): 685–690, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham NR, Kashikar-Zuck S, Mara CA, Goldschneider KR, Revicki DA, Dampier C, Sherry DD, Crosby L, Carle A, Cook KF, Morgan E: Development and validation of the self-reported PROMIS pediatric pain behavior item bank and short form scale. Pain 158(7): 1323–1331, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeWalt DA, Rothrock N, Yount S, Stone AA, on behalf of the PROMIS Cooperative Group: Evaluation of item candidates: The PROMIS qualitative item review. Med Care 45(5 Suppl 1): S12–21, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelen MO, Saliba D: Correspondence of verbal descriptor and numeric rating scales for pain intensity: An item response theory calibration. J Gerontol A Biol Sci Med Sci 65(7): 778–785, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Farrell JE, Kashikar-Zuck S, Jacobson CJ, Correia H, Dampier C, Verkamp E, Segerman J, Morgan E: The role of cognitive interviews in the development of pain behavior and pain quality item banks for pediatrics: A Patient-Reported Outcomes Measurement Information System (PROMIS) study. J Pain 13 (supplement): 124, 2012. [Google Scholar]
- 17.Hernandez-Boussard T, Graham LA, Desai K, Wahl TS, Aucoin E, Richman JS, Morris MS, Itani KM, Telford GL, Hawn MT: The fifth vital sign postoperative pain predicts 30-day readmissions and subsequent emergency department visits. Ann Surg 266(3): 516–524, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B: The Faces Pain Scale - Revised: Toward a common metric in pediatric pain measurement. Pain 93(2): 173–183, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hu LT, Bentler PM: Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 6(1): 1–55, 1999. [Google Scholar]
- 20.Huguet A, Miro J: The severity of chronic pediatric pain: An epidemiological study. J Pain 9(3): 226–236, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA: An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res 19(4): 595–607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin DE, Varni JW, Yeatts K, DeWalt DA: Cognitive interviewing methodology in the development of a pediatric item bank: a patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes 7: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson CJ, Farrell JE, Kashikar-Zuck S, Seid M, Verkamp E, Morgan-Dewitt E: Disclosure and self-report of emotional, social, and physical health in children and adolescents with chronic pain--a qualitative study of PROMIS pediatric measures. J Pediatr Psychol 38(1): 82–93, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson CJ Jr., Kashikar-Zuck S, Farrell J, Barnett K, Goldschneider K, Dampier C, Cunningham N, Crosby L, Morgan-DeWitt E: Qualitative evaluation of pediatric pain behavior, quality, and intensity item candidates and the PROMIS pain domain framework in children with chronic pain. J Pain 16(12): 1243–1255, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai JS, Stucky BD, Thissen D, Varni JW, Morgan-DeWitt E, Irwin DE, Yeatts KB, DeWalt DA: Development and psychometric properties of the PROMIS pediatric fatigue item banks. Qual Life Res 22(9): 2417–2427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maunuksela EL, Olkkola KT, Korpela R: Measurement of pain in children with self-reporting and behavioral assessment. Clin Pharmacol Ther 42(2): 137–141, 1987. [DOI] [PubMed] [Google Scholar]
- 27.McCaffery M: Nursing practice theories related to cognition, bodily pain, and man-environment interactions.: Los Angeles: UCLA Students’ Store; 1968. [Google Scholar]
- 28.McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC: A new analogue scale for assessing children’s pain: An initial validation study. Pain 64(3): 435–443, 1996. [DOI] [PubMed] [Google Scholar]
- 29.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L, PedImmpact: Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 9(9): 771–783, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Muthén LK, Muthén BO: Mplus User’s Guide. In. 8th ed. Los Angeles, CA: Muthén & Muthén; 1998–2017. [Google Scholar]
- 31.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D, on behalf of the PROMIS Cooperative Group: Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 45(5 Suppl 1): S22–31, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sheilds BJ, Palermo TM, Powers JD, Grewe SD, Smith GA: Predictors of a child’s ability to use a visual analogue scale. Child Care Health Dev 29: 281–290, 2003. [DOI] [PubMed] [Google Scholar]
- 33.van Laerhoven H, van der Zaag-Loonen HJ, Derkx BH: A comparison of Likert scale and visual analogue scales as response options in children’s questionnaires. Acta Paediatr 93(6): 830–835, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Varni JW, Magnus B, Stucky BD, Liu Y, Quinn H, Thissen D, Gross HE, Huang IC, DeWalt DA: Psychometric properties of the PROMIS pediatric scales: Precision, stability, and comparison of different scoring and administration options. Qual Life Res 23(4): 1233–1243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varni JW, Stucky BD, Thissen D, Morgan-DeWitt E, Irwin DE, Lai JS, Yeatts K, DeWalt DA: PROMIS pediatric pain interference scale: An item response theory analysis of the pediatric pain item bank. J Pain 11(11): 1109–1119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Korff M, Ormel J, Keefe FJ, Dworkin SF: Grading the severity of chronic pain. Pain 50(2): 133–149, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Wager J, Hechler T, Darlington AS, Hirschfeld G, Vocks S, Zernikow B: Classifying the severity of paediatric chronic pain - an application of the chronic pain grading. Eur J Pain 17(9): 1393–1402, 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.