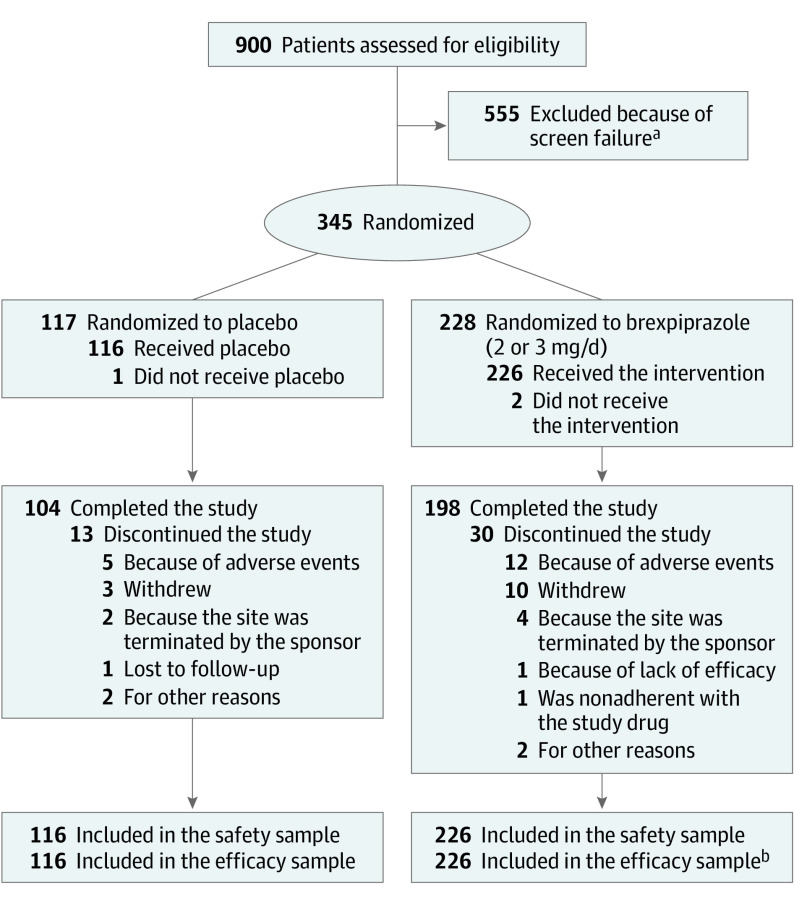
Figure 1. Patient Disposition.
aDefined as a patient who provided informed consent but was not randomized. The top 5 categories of screen failure were blinded Cohen-Mansfield Agitation Inventory (CMAI) factor 1 criterion, investigator or sponsor discretion, unstable diabetes, abnormal test results (laboratory tests, vital signs, electrocardiograms), and Mini-Mental State Examination score.
bIn the brexpiprazole group, the efficacy sample comprised 226 patients who took at least 1 dose of study drug and who had a baseline and postbaseline CMAI measurement. However, 1 patient was excluded from efficacy analyses because their only postbaseline CMAI measurement was outside of the visit window.