Figure 3.
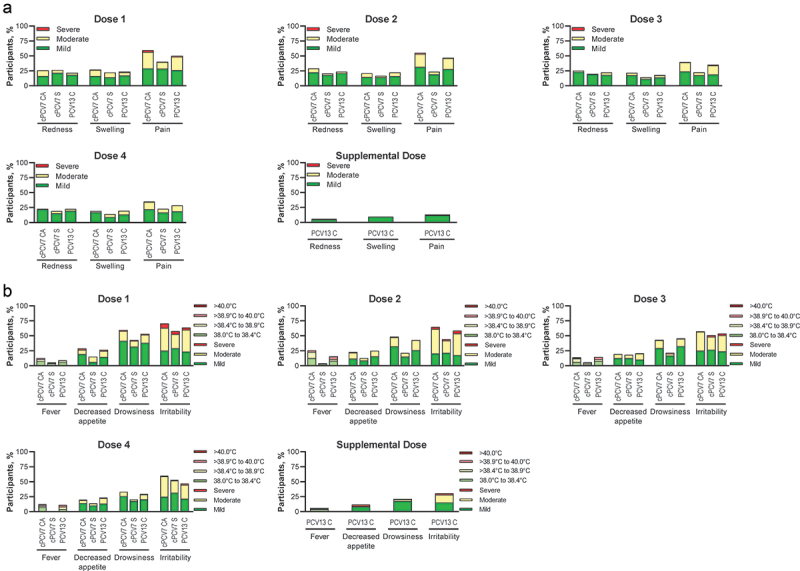
Participants reporting (a) local reactions and (b) systemic events within 7 days after Dose 1, Dose 2, Dose 3, Dose 4, and Supplemental Dose (safety population). Doses 1, 2, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. For redness and swelling, mild was > 0.0 to 2.0 cm, moderate was > 2.0 to 7.0 cm, and severe was >7.0 cm. Pain at the injection site was mild if it hurt when gently touched (eg, participant whimpered, winced, protested, or withdrew), moderate if it hurt when gently touched with crying, and severe if it caused limitation of limb movement. For decreased appetite, mild was decreased interest in eating, moderate was decreased oral intake, and severe was refusal to feed. For drowsiness, mild was increased or prolonged sleeping bouts; moderate was slightly subdued, interfering with daily activity; and severe was disabling, not interested in usual daily activity. For irritability, mild was easily consolable; moderate was requiring increased attention; and severe was inconsolable, crying cannot be comforted. The numbers of subjects included in the analyses were as follows: Dose 1, n = 144‒166 per group; Dose 2, n = 126‒152 per group; Dose 3, n = 120‒143 per group; Dose 4, n = 79‒105 per group; Supplemental Dose, n = 85 for the PCV13 Control group. C = control; CA = coadministered; cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; S = separated.
