Figure 5.
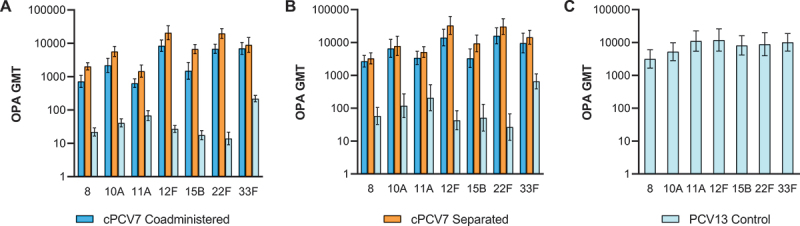
Serotype-specific OPA GMTs for the cPCV7 serotypes at (a) 1 month after Dose 3 (Dose 3 evaluable immunogenicity population), (b) 1 month after Dose 4 (Dose 4 evaluable immunogenicity population), and (c) 1 month after the Supplemental Dose (Supplemental Dose all-available immunogenicity population). Doses 3 and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. OPA titers were determined based on serum from a randomly selected subset of participants. Error bars display the upper and lower bounds of the 2-sided 95% CIs for GMTs calculated based on the Student t distribution. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMT calculations. At 1 month after Dose 3, n = 38‒46 for the cPCV7 Coadministered group, n = 38‒45 for the cPCV7 Separated group, and n = 40‒44 for the PCV13 Control group. At 1 month after Dose 4, n = 24‒28 for the cPCV7 Coadministered group, n = 25‒28 for the cPCV7 Separated group, and n = 24‒29 for the PCV13 Control group. At 1 month after the Supplemental Dose, n = 21‒26 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMT = geometric mean titer; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine.
