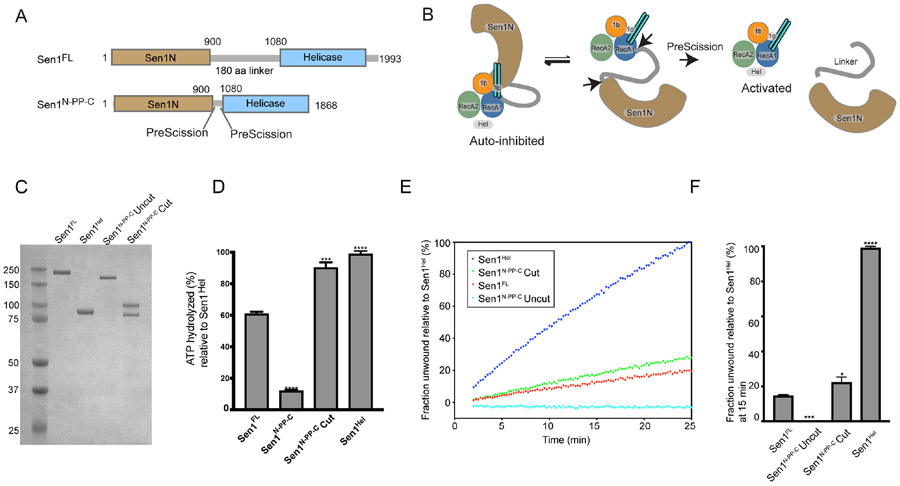
Figure 4. The Sen1N domain is autoinhibitory.
(A) Design of a PreScission Protease cleavable Sen1.
(B) Schematic of activation of PreScission Protease cleavable Sen1N-PP-C.
(C) Purified Sen1 proteins or PreScission Protease cleaved protein (Sen1N-PP-C – Cut) used in panels D–F.
(D) Substrate stimulated ATP hydrolysis of mutant Sen1 proteins was monitored in a phosphate release assay. Sen1FL, Sen1N-PP-C (Uncut), PreScission Protease-cleaved Sen1N-PP-C (Cut) and Sen1Hel (5 nM) were incubated with Sub6 (Supplementary Table 3) at 5 μM and ATP (1 mM) and MgCl2 (2 mM) for 15 min at 37 °C. Error bars are SD from 3 replicates, ***p<0.001, **** p<0.0001.
(E) Helicase assay of indicated Sen1 protein (1 nM) was performed as in Figure 2A and normalized to unwound fraction of Sen1Hel for comparison.
(F) Comparison of unwound fractions at 25 min from experiments in “E”. Error bars are SD from 3 replicates. Statistical analysis was done using paired t-test relative to Sen1FL. *p<0.05, ***p<0.001, ****p<0.0001.