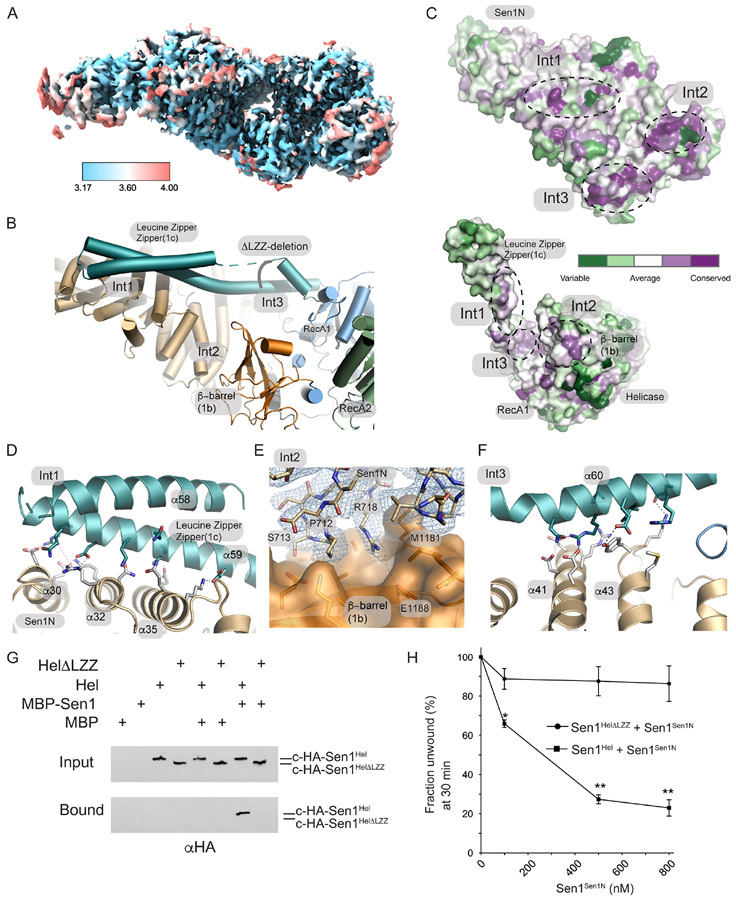
Figure 5. Cryo-EM structure of Sen1N-PP-C.
(A) Local resolution of Sen1N-PP-C, calculated in using the Local Resolution Estimation in CryoSparc, and displayed using a blue (high-resolution, 3.17 Å) to red (low-resolution, 4.0 Å) color-coded surface.
(B) Three interaction interfaces (Int1-Int3) mediate Sen1Sen1N interaction with Sen1Hel. The LZZ from Sen1Hel binds both Int1 and Int3 on Sen1Sen1N. A ΔLZZ internal deletion construct is marked.
(C) Consurf analysis of the Sen1Sen1N surface reveals conserved surface interaction interfaces (Int1-Int3, dotted lines) are involved in interactions with Sen1Hel. Top: Sen1N domain interaction interfaces that bind the helicase domain. Bottom: Helicase domain interaction surfaces that bind the Sen1N domain.
(D) Molecular details of the Int1 interdomain Sen1Sen1N - Sen1Hel interaction interface.
(E) Cryo-EM density for Sen1Sen1N Int2 region displayed contoured at 11.0 σ overlaid upon a molecular surface diagram of the β-barrel domain (orange).
(F) Molecular details of the Int3 interdomain Sen1Sen1N - Sen1Hel interaction interface.
(G) MBP-pulldowns show the LZZ is critical for the Sen1Sen1N–Sen1Hel interaction.
(H) Trans inhibition of Sen1Hel by Sen1Sen1N. Sen1Hel (1 nM) was pre-incubated with Sen1Sen1N at the indicated concentrations for 5 min on ice. Reactions were initiated by addition of 50 nM Sub7 (Supplementary Table 3) and a mixture containing ATP (1 mM) and MgCl2 (2 mM). The percentage of unwound ssDNA was calculated from the FAM signal at 30 min. Error bars are SD from 3 replicates, **p<0.01, *p<0.05.