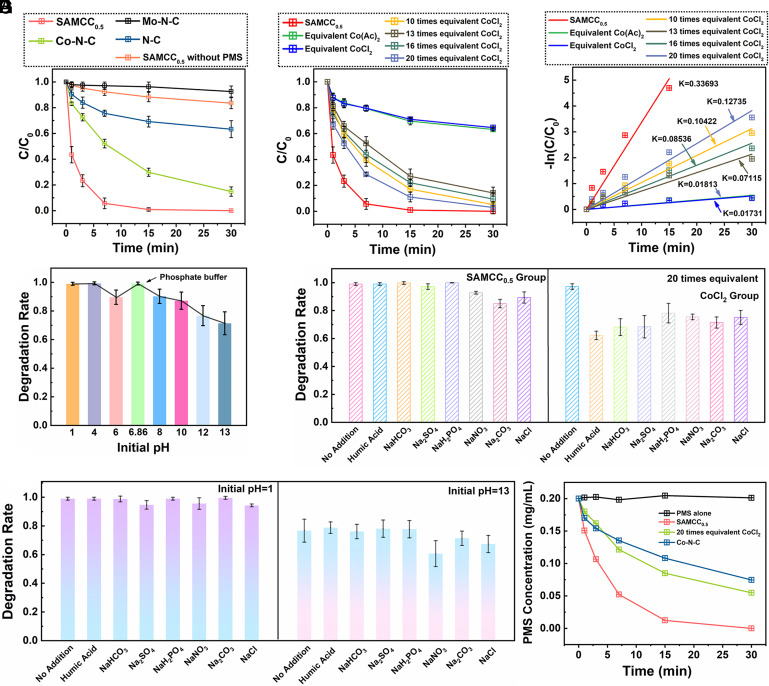
Fig. 2.
(A) Degradation rate of phenol when SAMCC0.5, Co–N–C, Mo–N–C and N–C were used as catalysts. (B) Degradation rate of phenol when 300 mg L−1 SAMCC0.5 and different equivalents of CoCl2 were used as catalysts. (C) Apparent kinetic constants of (B). (D) SAMCC0.5 catalytic degradation rate of phenol under different pH environments. (E) SAMCC0.5 and different equivalents of CoCl2 catalytic degradation rate of phenol with interference of 0.2 M salts and humic acid. (F) SAMCC0.5 catalytic degradation rate of phenol with interference of 0.2 M salts and humic acid with pH = 1 and 13. (G) Decomposition rate of PMS with the catalytic effect of SAMCC0.5, Co–N–C and 20 times equivalent CoCl2. Dosage: PMS: 200 mg L−1, reaction solution: 100 mL, catalyst: 300 mg L−1, reaction time: 30 min.