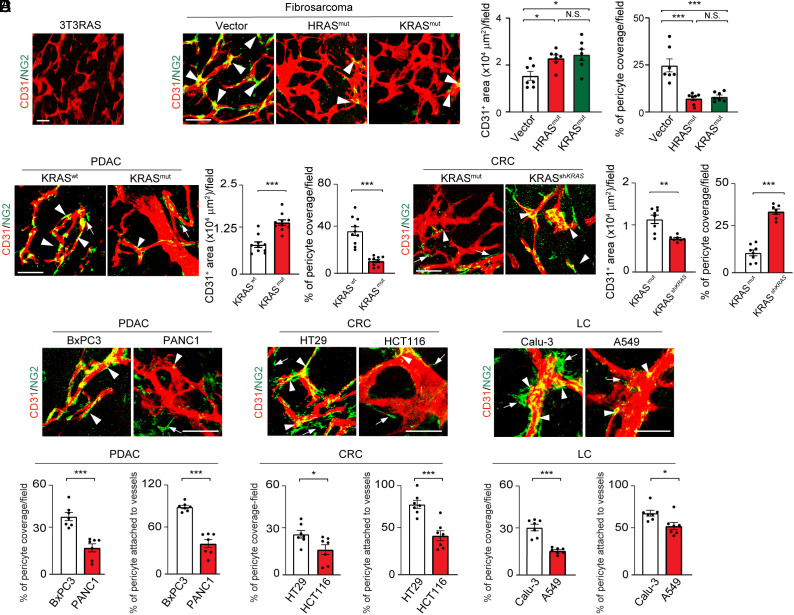
Fig. 1.
Mutant RAS tumors promote pericyte ablation in tumor vasculatures. (A) CD31+ tumor microvessels (red) and NG2+ pericytes (green) in murine 3T3RAS tumor. (B) CD31+ tumor microvessels (red) and NG2+ pericytes (green) in murine T241-vector, T241-HRAS mutant, and T241-KRAS mutant tumors. Quantification of microvessel density and pericyte coverage (n = 7 random fields per group). (C) Tumor microvessels (red) and pericytes (green) in human BxPC3 WT and BxPC3 KRAS mutant tumors. Quantification of microvessel density and vascular coverage by pericytes (n = 10 random fields per group). (D) Tumor microvessels (red) and pericytes (green) in human HCT116 KRAS mutant and HCT116 shKRAS tumors. Quantification of microvessel density and vascular coverage by pericytes (n = 7 to 8 random fields per group). (E) Tumor microvessels (red) and pericytes (green) in BxPC3 and PANC1 human PDAC tumors, HT29 and HCT116 human CRC tumors, and Calu-3 and A549 human lung tumors. Quantification of pericyte coverage and vessel-associated pericytes (n = 7 random fields per group). Arrowheads indicate vessel-associated pericytes, and arrows indicate pericytes disassociated from tumor vessels. Scale bar, 50 μm in all images. All data represent mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison tests (B) and two-sided unpaired t tests (C–E). *P < 0.05, **P < 0.01, ***P < 0.001. N.S., not significant. PDAC, pancreatic ductal adenocarcinoma; CRC, colorectal carcinoma; LC, lung cancer.