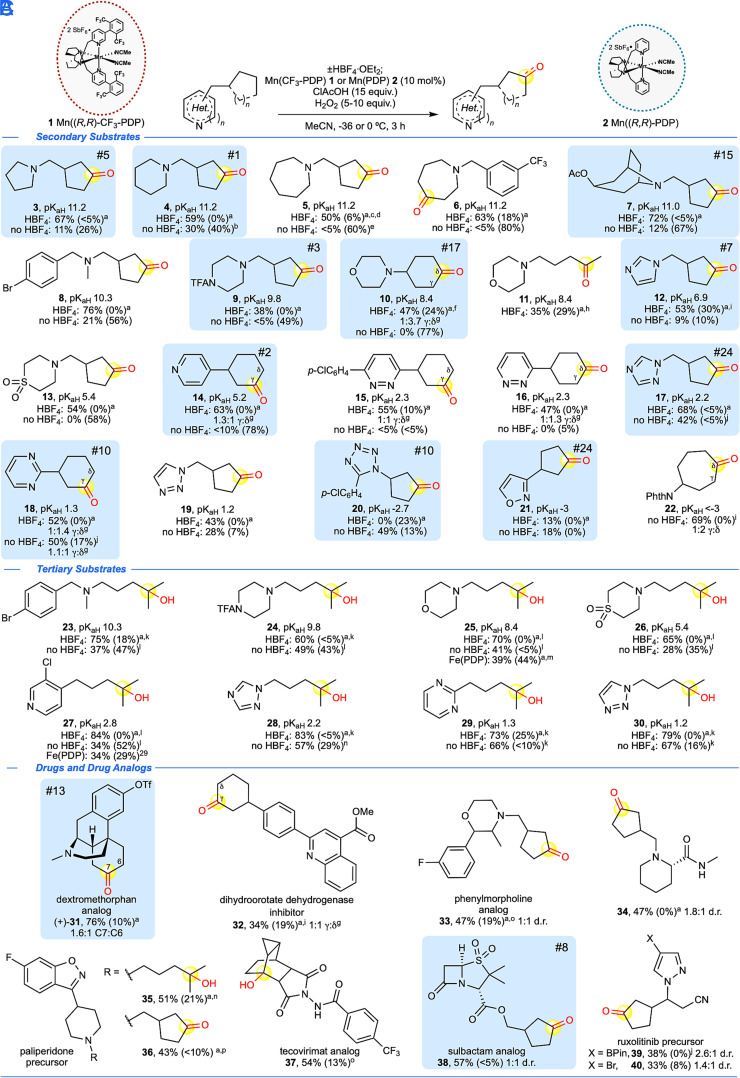
Fig. 2.
N-heterocycle scope of (A) Mn(CF3-PDP) 1 methylene C–H oxidation and (B) Mn(PDP) 2 tertiary C–H oxidations with and without HBF4 nitrogen protonation. (C) Aliphatic C–H oxidation of drugs and drug derivatives with and without HBF4 nitrogen protonation. Isolated yields are an average of two to three experiments, major isolated site of oxidation is highlighted in yellow, and recovered starting material (RSM) is given in parentheses. Experimental pKaH reported in water (see SI Appendix for references). Blue boxes with numbers indicate frequency of N-heterocycles in FDA approved drugs according to ref. 1. ClAcOH, chloroacetic acid. NPhth, phthalimide. General methylene oxidation with HBF4 was done using Method A at -36 °C unless otherwise noted. General methylene oxidation without HBF4 was done using Method B unless otherwise noted. aNitrogen was HBF4•OEt2 protected as described in Materials and Methods. bMethod A at 0 °C. cModified Method D [2.5 mol% Mn(CF3-PDP) 1, 7.5 equiv. ClAcOH, 5 equiv. H2O2] at -36 °C. dWith 20% di-oxidation. eModified Method D [2.5 mol% Mn(CF3-PDP) 1, 7.5 equiv. ClAcOH, 5 equiv. H2O2] at 0 °C. fMethod C [2 x 5 mol% Mn(CF3-PDP) 1] at 0 °C. gRatios are statistically corrected. hMethod C [3 x 5 mol% Mn(CF3-PDP) 1] at 0 °C. iMethod D. jMethod A at -36 °C. kMethod B with Mn(PDP) 2. lMethod A with Mn(PDP) 2. m3 x 5 mol% Fe(PDP), iterative addition protocol (ref. 21). nMethod C [2 x 5 mol% Mn(PDP) 2] at -36 °C. oMethod B. pMethod C [3 x 5 mol% Mn(CF3-PDP) 1] at -36 °C.