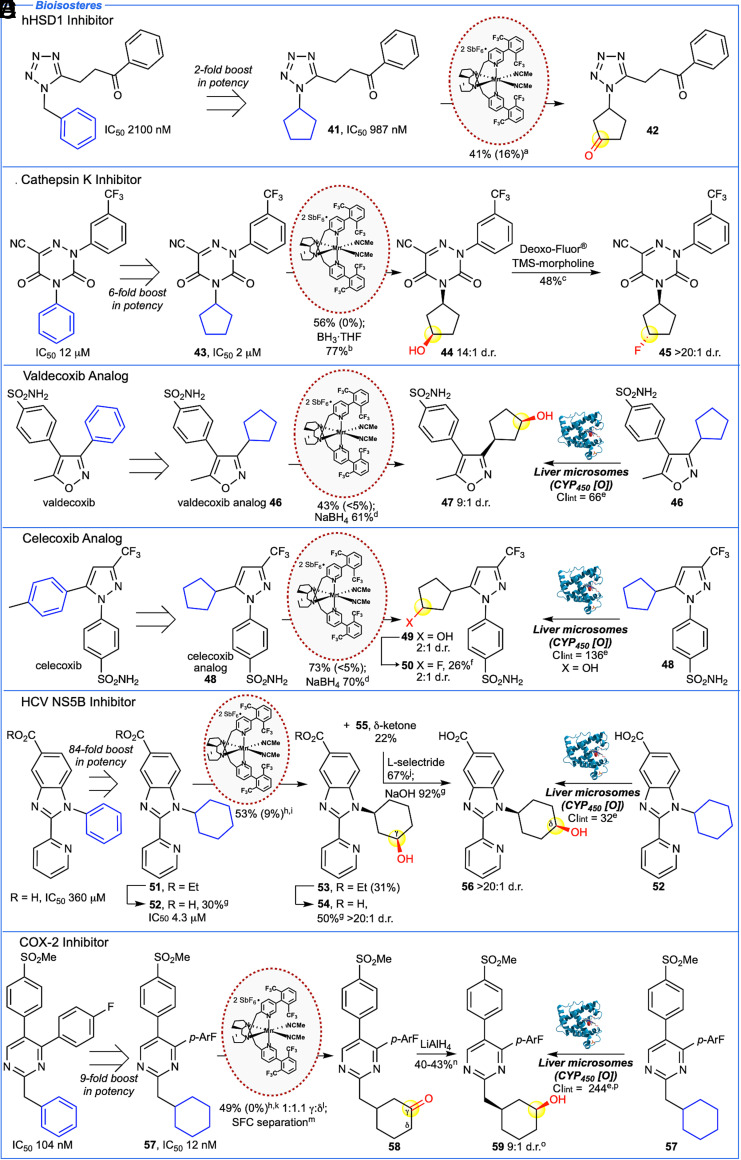
Fig. 3.
Mn(CF3-PDP) 1 C–H oxidation of saturated carbocycle bioisosteres of N-heterocyclic drug candidates. (A) 11β-hydroxysteroid dehydrogenase (hHSD1) inhibitor reported a twofold increase in potency for cyclopentyl bioisostere 41 that can be oxidized selectively to give cyclopentanone 42. (B) Cathepsin K inhibitor reported a sixfold increase in potency in cyclopentyl bioisostere 43 that can be oxidized selectively and diversified by reduction and deoxyfluorination. (C) Cyclopentyl bioisostere of valdecoxib 46 undergoes selective oxidation and reduction to form alcohol 47 at the same carbon as CYP450 enzymes. (D) Cyclopentyl bioisostere of celecoxib 48 undergoes selective oxidation and reduction to form alcohol 49 at the same carbon site as CYP450 enzymes. Fluorination affords 50 that shows reduced metabolism in rat liver microsomes (Clint 57 μL/min/mg) relative to 48. (E) Hepatitis C (HCV) inhibitor reported an 84-fold increase in potency in cyclohexyl bioisostere 52. Precursor 51 can be oxidized to form a single diastereomer of γ-alcohol 53 and δ-ketone 55. Reduction of the ketone and hydrolysis forms alcohol 56 at the same carbon site with the same stereochemistry as CYP450 enzymes. (F) COX-2 inhibitor reported a ninefold increase in potency in cyclohexyl bioisostere 57 that can be oxidized to give γ- and δ-ketones 58 which can be diversified by reduction to form alcohol 59 at the same carbon site as CYP450 enzymes. Method B is used unless otherwise noted. aMethod A at 0 °C. bBH3•THF (1.1 equiv.) in THF, 0 °C. cDeoxo-Fluor® (6.0 equiv.), TMS-morpholine (6.0 equiv.), refluxed in CH2Cl2 overnight. dNaBH4 (1.1 equiv.) in MeOH, 0 °C. eUnscaled intrinsic clearance in rat liver microsome preparations, μL of compound metabolized per minute per mg of protein. For human liver microsome preparations, see SI Appendix. fDeoxo-Fluor® (6.0 equiv.), TMS-morpholine (6.0 equiv.), refluxed in toluene overnight. g2M NaOH (2.7 equiv.) in MeOH, 60 °C. hNitrogen was HBF4•OEt2 protected. iMethod C [3 × 5 mol% Mn(CF3-PDP) 1] at −36 °C. Starting material recycled once. jL-selectride® (1.0 equiv.) in THF, −78 °C. kModified method A [5 mol% Mn(CF3-PDP) 1] at −36 °C. lRatios are statistically corrected. mKetone regioisomers/enantiomers separated by chiral SFC separation. nLiAlH4 (1.1 equiv.) in THF, −78 °C. oOptical rotation based on a 9:1 mixture of diastereoisomers. pUnscaled intrinsic clearance in human liver microsome preparations: 223 μL/min/mg.