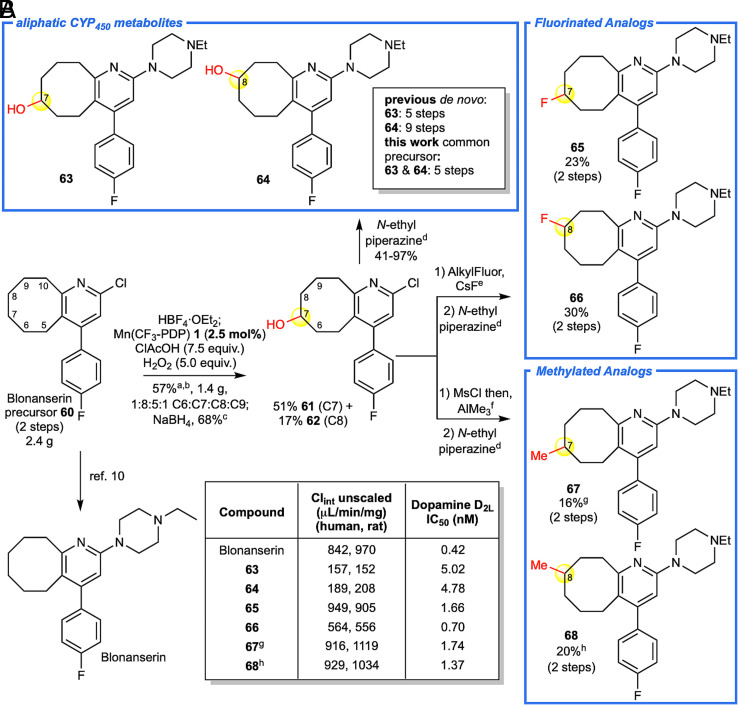
Fig. 4.
Streamlining blonanserin metabolite and analog synthesis. (A) Blonanserin precursor 60 was oxidized on a 2.4 g scale with Mn(CF3-PDP) 1 to afford 1.4 g of a mixture of ketone products at C7 and C8, known sites of CYP450 metabolism, with minor ketone products observed at C6 and C9. The yield is comparable to that on a 0.3-mmol scale using 10 mol% Mn(CF3-PDP) 1 (49% of C7 and C8). Reduction of ketones to alcohols 61 and 62 followed by SNAr cross-coupling afforded known metabolites 63 and 64. Diversification of alcohols 61 and 62 via deoxyfluorination and oxidative methylation followed by cross-coupling afforded fluorinated (65 and 66) and methylated (67 and 68) analogs, respectively. (B) Compounds from analog library were subjected to metabolism studies to measure intrinsic clearance in rat and human liver microsomes. Measurement of IC50 values of blonanserin and analogs against Dopamine D2L receptor using a known radioligand binding assay. aNitrogen was HBF4•OEt2 protected, and modified method D was used. bC7 and C8 were the major sites of oxidation, C7+C8:C6+C9 = 5.5:1. cNaBH4 (1.1 equiv.) in MeOH. dN-ethyl piperazine (15.0 equiv.), KI (1.0 equiv.), heated to 165 °C. eAlkylFluor (1.2 equiv.), CsF (5.0 equiv.) generate PhenoFluor in situ as described in ref. 62. fMethylation procedure as described in ref. 47 was performed on pure C7 and C8 alcohols. This afforded olefin by-products that were removed via HBF4•OEt2 nitrogen protection then mCPBA (1.6 equiv.) oxidation. g1:1 C7:C6. h2:1 C8:C9.