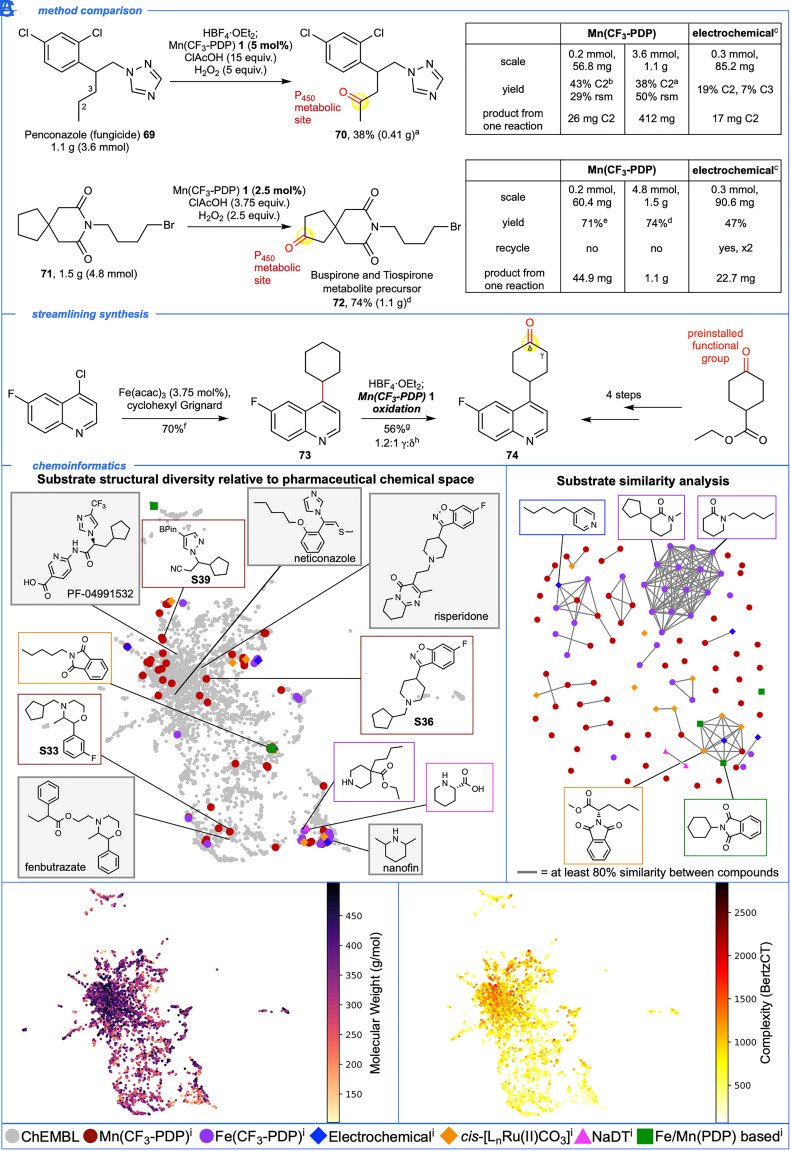
Fig. 5.
Benchmarking Mn(CF3-PDP) 1 oxidation to known chemoselective methylene C–H oxidations via experimental and chemoinformatic analysis. (A) Large-scale (1 to 1.5 g) oxidation of 69 and 71 with Mn(CF3-PDP) 1 at low catalyst loadings (2.5 to 5 mol%) affords known penconazole metabolite 70 and buspirone/tiospirone metabolite precursor 72 in preparative quantities (0.41 to 1.1 g). This compares favorably to previously reported electrochemical oxidations. (B) Mn(CF3-PDP) 1 oxidation enables streamlining synthesis of 74 en route to linrodostat (BMS-986205). (C) Chemoinformatic investigation of Mn(CF3-PDP) 1 and all other methods reporting methylene oxidation in N-heterocyclic compounds relative to pharmaceutical chemical space. All examples with ≥15% yield of mono-oxidized compounds were included. Pyrrolidine, piperidine, and azepane from NaDT (sodium decatungstate) were filtered out due to low molecular weight (<100 g/mol). aNitrogen was HBF4•OEt2 protected followed by modified method D at 0 °C. bNitrogen was HBF4•OEt2 protected followed by method A at 0 °C. cFrom ref. 38. dMethod D. eMethod B. fQuinoline (1.05 equiv.), Fe(acac)3 (3.75 mol%) and cyclohexyl Grignard (1.0 equiv.) in THF/NMP at rt for 75 min as described in ref. 66. gNitrogen was HBF4•OEt2 protected followed by method A. hRatios are statistically corrected. iThese catalysts can be found in refs. 26, 29, 36–42.