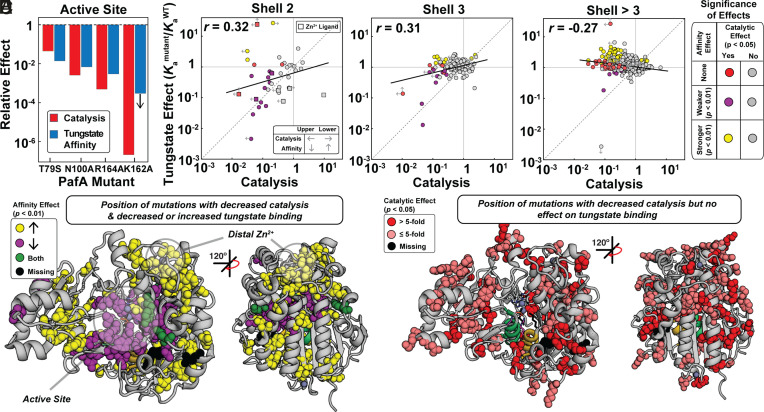
Fig. 4.
Effects of mutations throughout the PafA structure on tungstate binding and catalysis as a function of distance from the PafA active site. (A) Comparison of catalytic and tungstate affinity effects of PafA active-site mutants, measured previously (51). (B–D) Comparison of catalytic and tungstate affinity effects for mutants of residues in the (B) second shell, (C) third shell, and (D) more distal shells. Colored and gray points denote statistically significant and insignificant effects, respectively, based on statistical tests (bootstrap hypothesis tests) comparing each mutant to WT PafA. Left arrows denote upper limits for mutants with catalytic activities below the dynamic range of the assay, right arrows denote lower limits for mutants with MeP KM values below the lowest substrate concentration used, and vertical arrows denote upper and lower tungstate Ka limits (Materials and Methods). (E) PafA structure showing locations of residues with significant catalytic and either deleterious (28 of 522 measurable residues) or enhanced (57 of 522 residues) tungstate affinity effects when mutated to either glycine or valine. (F) PafA structure showing positions in PafA that have significant catalytic effects but do not have significant affinity effects (80 of 522 residues). Catalytic data are from reference (50).