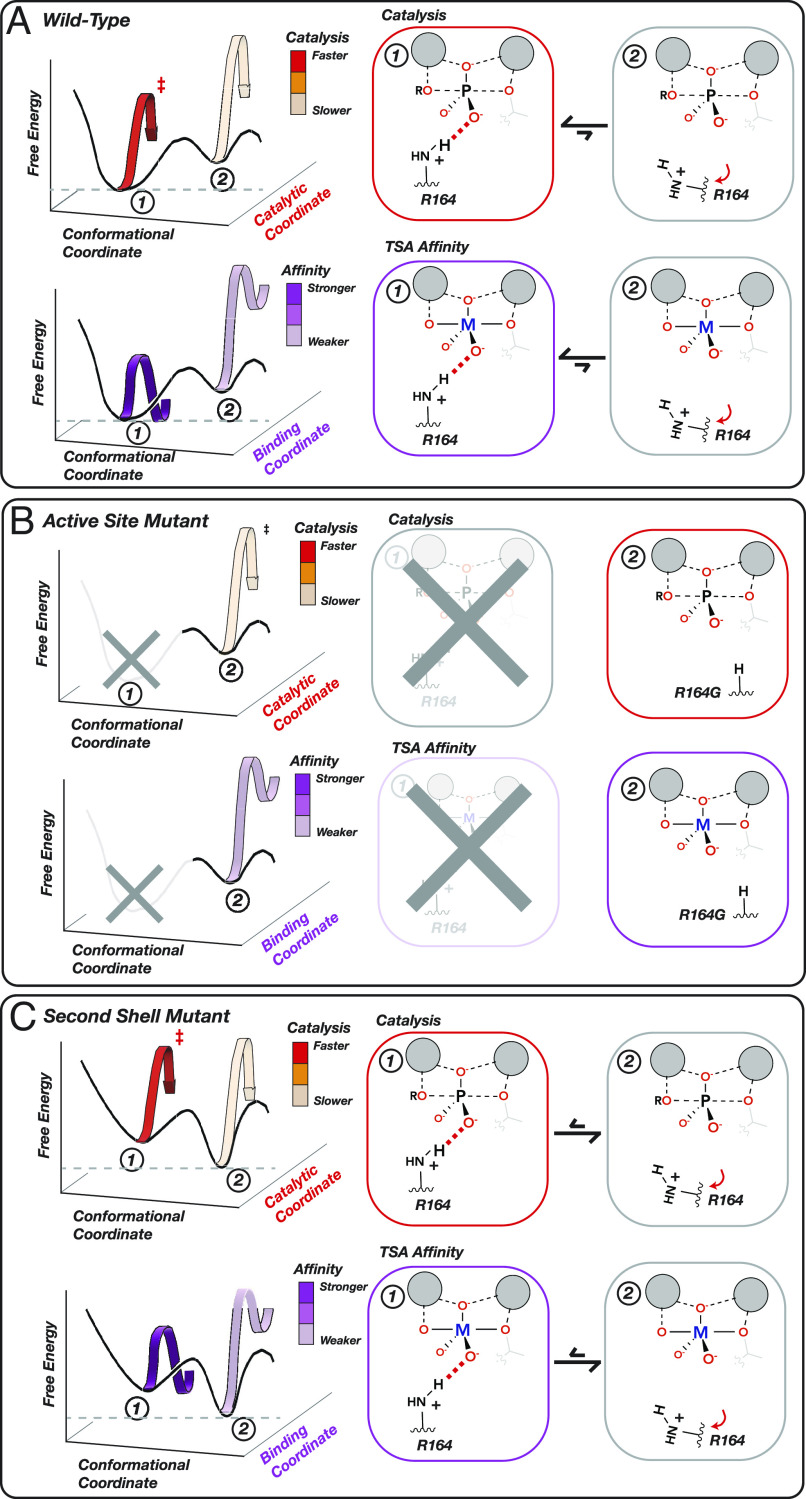
Fig. 6.
Physical models accounting for observed effects of mutating active-site and second-shell residues. (A) A WT PafA active-site residue shown in a conformational equilibrium between two states. In State 1 (favored), R164 donates a hydrogen bond to an oxygen atom in the TS and TSA, promoting catalysis and TSA binding (M = V, W). In State 2 (disfavored), R164 is mispositioned so that the hydrogen bond no longer forms, reducing both catalysis and TSA binding. (B) Ablation of the R164 side chain eliminates State 1, reducing catalysis and TSA binding to the same extent [to the level of WT State 2 alone (A)]. (C) Mutating a second-shell residue that positions R164 destabilizes State 1 and increases occupancy of State 2. This change in the conformational equilibrium equally reduces catalysis and TSA binding, proportional to the increased occupancy of State 2.