Fig. 7.
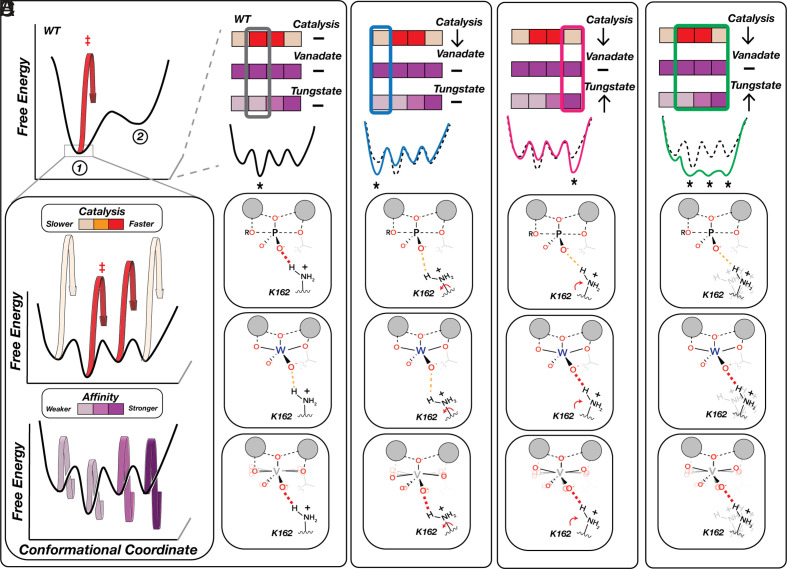
Physical models for the impacts of distal residue mutations that decouple catalytic and TSA affinity effects. (A) WT-favored State 1 is itself an ensemble of microstates (that is, states with smaller conformational changes than illustrated for the mutants in Fig. 6) with different levels of catalysis (Middle) and TSA binding (Bottom), denoted by color bars. The most populated WT microstate is optimal for catalysis but not for tungstate binding, whereas vanadate’s conformational flexibility allows the ligand to adopt more optimal geometries in each microstate. (B–D) Examples of distal mutational effects with differential impacts on catalysis and TSA binding due to alternation in microstate distributions. (B) Stabilization of a less catalytically competent microstate that maintains WT TSA affinities (blue). (C) Stabilization of a less catalytically competent microstate that preferentially binds the distorted tungstate geometry (pink). (D) Flattening of the conformational landscape due to increased flexibility results in similar occupancies of multiple microstates, including some with increased affinity for the distorted geometry of tungstate (green). Not shown are distal effects that phenocopy the effects described in Fig. 6B. For simplicity we show changes that do not alter vanadate affinity; additional states are required to account for mutants that alter vanadate affinity the same or differently than catalysis and tungstate affinity.