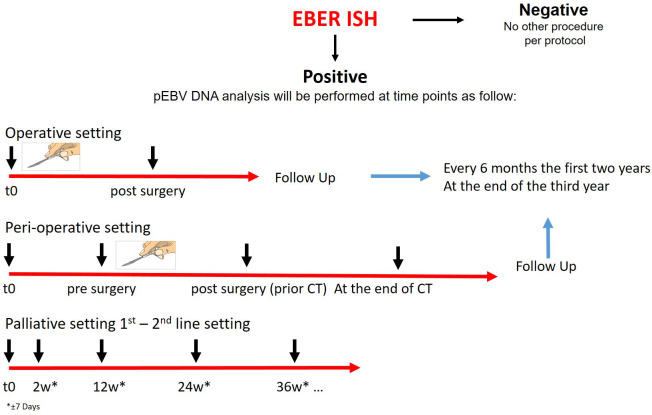
Figure 1.
Study design. CT chemotherapy, EBV Epstein Barr virus, EBER Epstein-Barr Encoding Region, ISH in situ hybridization. Patients with GC will be screened with EBER ISH. If EBER ISH is positive, blood analysis for plasma EBV DNA will be conducted. Plasma EBV DNA analysis will be performed according to the setting at different times. During follow up blood samples will be collected every 6 months the first two years and at the end of the third year.