FIGURE.
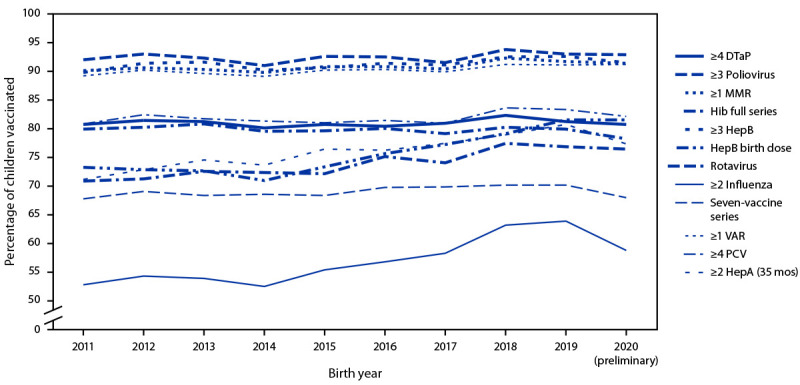
Estimated coverage with selected individual vaccines*,†,§,¶,**,††,§§ and a combined vaccine series¶¶ by age 24 months, by birth year*** — National Immunization Survey-Child, United States, 2012–2022
Abbreviations: DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; HepA = hepatitis A vaccine; HepB = hepatitis B vaccine; Hib = Haemophilus influenzae type b conjugate vaccine; MMR = measles, mumps, and rubella vaccine; PCV = pneumococcal conjugate vaccine; VAR = varicella vaccine.
* Includes vaccinations received by age 24 months, except for the HepB birth dose, rotavirus vaccination, and ≥2 HepA doses by 35 months. For all vaccines except the HepB birth dose and rotavirus vaccination, the Kaplan-Meier method was used to estimate vaccination coverage to account for children whose vaccination history was ascertained before age 24 months (35 months for ≥2 HepA doses).
† Includes children who might have been vaccinated with diphtheria and tetanus toxoids vaccine or diphtheria, tetanus toxoids, and pertussis vaccine.
§ Includes children who might have been vaccinated with MMR and varicella combination vaccine.
¶ Hib full series: primary series and booster dose, which includes receipt of ≥3 or ≥4 doses, depending on product type received.
** One dose HepB administered from birth through age 3 days.
†† Includes ≥2 doses of Rotarix monovalent rotavirus vaccine or ≥3 doses of RotaTeq pentavalent rotavirus vaccine; if any dose in the series is either RotaTeq or unknown, the default is to a 3-dose series. The maximum age for the final rotavirus dose is 8 months, 0 days.
§§ Influenza vaccine doses must be ≥24 days apart (4 weeks with a 4-day grace period); doses could have been received during two influenza seasons.
¶¶ The combined seven-vaccine series (4:3:1:3*:3:1:4) includes ≥4 doses of DTaP, ≥3 doses of poliovirus vaccine, ≥1 dose of measles-containing vaccine, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
*** Children born in 2011 are included in survey years 2012, 2013, and 2014; children born in 2012 are included in survey years 2013, 2014, and 2015; children born in 2013 are included in survey years 2014, 2015, and 2016, children born in 2014 are included in survey years 2015, 2016, and 2017; children born in 2015 are included in survey years 2016, 2017, and 2018; children born in 2016 are included in survey years 2017, 2018, and 2019; children born in 2017 are included in survey years 2018, 2019 and 2020; children born in 2018 are included in survey years 2019 and 2020, and 2021; children born in 2019 are included in survey years 2020, 2021, and 2022; data for children born in 2020 are considered preliminary and are from survey years 2021 and 2022 (data from survey year 2023 are not yet available).
