Abstract
In unconscious appearing patients with acute brain injury, wilful brain activation to motor commands without behavioural signs of command following, known as cognitive motor dissociation (CMD), is associated with functional recovery. CMD can be detected by applying machine learning to EEG recorded during motor command presentation in behaviourally unresponsive patients. Identifying patients with CMD carries clinical implications for patient interactions, communication with families, and guidance of therapeutic decisions but underlying mechanisms of CMD remain unknown.
By analysing structural lesion patterns and network level dysfunction we tested the hypothesis that, in cases with preserved arousal and command comprehension, a failure to integrate comprehended motor commands with motor outputs underlies CMD. Manual segmentation of T2-fluid attenuated inversion recovery and diffusion weighted imaging sequences quantifying structural injury was performed in consecutive unresponsive patients with acute brain injury (n = 107) who underwent EEG-based CMD assessments and MRI. Lesion pattern analysis was applied to identify lesion patterns common among patients with (n = 21) and without CMD (n = 86). Thalamocortical and cortico-cortical network connectivity were assessed applying ABCD classification of power spectral density plots and weighted pairwise phase consistency (WPPC) to resting EEG, respectively.
Two distinct structural lesion patterns were identified on MRI for CMD and three for non-CMD patients. In non-CMD patients, injury to brainstem arousal pathways including the midbrain were seen, while no CMD patients had midbrain lesions. A group of non-CMD patients was identified with injury to the left thalamus, implicating possible language comprehension difficulties. Shared lesion patterns of globus pallidus and putamen were seen for a group of CMD patients, which have been implicated as part of the anterior forebrain mesocircuit in patients with reversible disorders of consciousness. Thalamocortical network dysfunction was less common in CMD patients [ABCD-index 2.3 (interquartile range, IQR 2.1–3.0) versus 1.4 (IQR 1.0–2.0), P < 0.0001; presence of D 36% versus 3%, P = 0.0006], but WPPC was not different. Bilateral cortical lesions were seen in patients with and without CMD. Thalamocortical disruption did not differ for those with CMD, but long-range WPPC was decreased in 1–4 Hz [odds ratio (OR) 0.8; 95% confidence interval (CI) 0.7–0.9] and increased in 14–30 Hz frequency ranges (OR 1.2; 95% CI 1.0–1.5).
These structural and functional data implicate a failure of motor command integration at the anterior forebrain mesocircuit level with preserved thalamocortical network function for CMD patients with subcortical lesions. Amongst patients with bilateral cortical lesions preserved cortico-cortical network function is associated with CMD detection. These data may allow screening for CMD based on widely available structural MRI and resting EEG.
Keywords: cognitive motor dissociation, electroencephalography, imaging, MRI, structural injury
Franzova and Shen et al. report that unresponsive patients with cognitive motor dissociation have intact ascending arousal pathways, preserved thalamocortical function, and speech comprehension, but unique patterns of structural brain injury implicating a failure of motor command integration.
Introduction
Disorders of consciousness (DoC) are common amongst patients with acute brain injury but underlying mechanisms, trajectory of recoveries, and targeted treatments are only starting to emerge.1,2 In intensive care units (ICUs) around the world, consciousness is assessed by asking patients to perform motor acts, such as ‘show me two fingers’, often many times each day. These unassuming appearing commands, also labelled as ‘following simple commands’, require intact function of large areas of the nervous system, which support wakefulness, attention, speech comprehension, motor planning with access to motor programmes, enacting these motor programmes with adjustments based on the context, and movements of body parts.3 The neural systems supporting these functions do not only have to work in isolation but also need to be highly coordinated, requiring large scale interactions that probe the function of much of the brain.4,5 Lesion studies have a long tradition to provide first insights into potential mechanisms of neuropathological phenomena.6
DoCs have been categorized based on clinical examination findings into states without behavioural evidence of command following such as coma and vegetative state and those with discernible signs of consciousness with or without residual language function, such as minimally conscious state (MCS) minus and plus, respectively.7 Advanced neuroimaging and electrophysiological techniques have revealed that brain function cannot comprehensively be captured by behavioural assessments alone. It has become clear that new terminology is required for patients who exhibit no behavioural signs of consciousness at the bedside but have evidence of brain activation on advanced imaging and EEG testing.3,8–12 A number of terms including non-behavioural MCS, MCS*, functional locked-in syndrome, and covert cortical processing have been used to describe related but slightly different phenomena.1,13–16 For a comprehesive review on definitions and distinctions between these, refer to Schnakers et al.17 Here we conceptualize the dissociation of behaviour and brain function using the term cognitive motor dissociation (CMD) to describe patients with evidence of command following on functional MRI (fMRI) or EEG without behavioural evidence of command following.17,18 Behaviourally these patients may be comatose, in a vegetative state or unresponsive wakefulness syndrome, or in a minimally conscious state minus.19 CMD has been reported in 15–25% of unresponsive patients, including those with severe acquired brain injury, including traumatic brain injury (TBI), brain haemorrhages, and cardiac arrest.8,18–20 CMD has been associated with a higher chance of early recovery of behavioural evidence of consciousness and good neurological function at 1 year after injury.8,19–21 It is proposed that detection of CMD may allow more precise characterization of the individual patient endotype enabling more precise clinical trajectory predictions.8,22
The underlying mechanisms of CMD are poorly understood and the phenomenon remains puzzling considering that these patients are able to hear, have distinct brain activation to commands, and are not paralysed, yet they do not act out the commands. Even though behaviourally these patients are by definition indistinguishable from non-CMD patients, EEG organization and features of sleep architecture of CMD patients may more closely resemble those of command-following patients.19 Metabolic activity as evidenced by PET scanning is preserved in CMD patients with command-following revealed by fMRI.23
Linking structural injury patterns to standardized assessments of brain function may yield mechanistic insights for CMD as it has done for other DoC.24,25 Among a previously published cohort of acutely brain injured patients with EEG-detected CMD, no obvious or unifying structural brain lesion was identified but in a case-of-one study, selective structural disruption between the thalamus and the primary motor cortex has been implicated for the failure of motor execution with preserved motor imagery.19,26 Structural connectivity is increased in patients with CMD when compared to those without and behavioural evidence of command following may be elicited in these patients following stimulation of the dorsolateral prefrontal cortex.27,28 Patients with subtle behavioural evidence of command following detected only by the Motor Behavior Tool-revised (MBT-r) had evidence of structural injury in the left mesencephalon, right basal ganglia, right thalamus, right parietal cortex and left frontal cortex.29,30 Network level dysfunction assessed by resting state EEG analysis may additionally provide insights into functional connectivity underlying CMD.22,31
In the current study, we tested the hypothesis that in cases with preserved arousal and command comprehension based on EEG, a failure to integrate comprehended motor commands with motor outputs during behavioural assessments underlies CMD. This hypothesis builds on the notion that recruitment of widely distributed networks is necessary to comprehend and execute motor commands.32 We used a cohort of patients that was carefully assessed for CMD using a widely accepted EEG paradigm19,20,31,33 and underwent structural brain imaging by MRI and analysed resting state EEG.
Materials and methods
Patient cohort
Between July 2014 and January 2021 all patients with acute brain injury that were admitted to the Neuroscience ICU at New York Presbyterian Hospital—Columbia University were prospectively screened for the inability to follow commands. We considered all patients (i) with acute brain injury; that were (ii) found to be in a coma, vegetative state, or minimally conscious state minus as defined by the Coma Recover Scale-Revised (CRS-R); (iii) that were then assessed for CMD using an EEG recorded motor command protocol; and (iv) that underwent brain MRI during their acute hospitalization for this study.19 Patients were excluded if (i) the MRI was not obtained during the hospitalization, if both diffusion weighted imaging (DWI) and fluid attenuated inversion recovery (FLAIR) sequences were not obtained or uninterpretable, or if artefacts precluded identification of anatomical regions of interest (ROIs) (Supplementary Fig. 1).30 We also excluded all patients who were (ii) less than 18 years of age; (iii) had a pre-existing disorder of consciousness or confounding neurological condition (i.e. baseline aphasia or advanced dementia) prior to the onset of their presenting acute brain injury; (iv) were deaf prior to the acute brain injury; (v) did not speak English or Spanish as their first language; (vi) clinically recovered the ability to follow commands prior to enrolment; (vii) did not want to participate or whose family did not want them to participate; (viii) had uncontrollable seizures; or (ix) had logistical reasons that hindered their enrolment.
EEG recording
EEG was recorded using a digital bedside video EEG monitoring system (XLTEK, Excel-Tech Corp., Natus Medical Incorporated; low-pass filter = 70 Hz, high-pass = 1 Hz, sampling rate = 200 Hz) with 21 EEG electrodes placed according to the International 10–20 system. Each EEG assessment was preceded by a clinical examination that included the CRS-R in order to categorize the clinical state of consciousness at the time of the recording. In addition to twice-daily lead maintenance by EEG technicians, research coordinators and study physicians were trained to recognize lead artefacts and other frequently encountered artefacts to trigger lead maintenance with the goal to optimize EEG quality prior to and during recordings. Research coordinators continuously monitored the raw online EEG while recording the commanded motor act trials and if artefact was detected (e.g. movement, cough) were instructed to repeat the recording of the affected block.
Detection of CMD
Spoken command instructions during EEG recordings alternated between ‘keep opening and closing your right hand’ and ‘stop opening and closing your right hand’. A total of six blocks each with eight consecutive trials of ‘keep opening …’ and ‘stop opening …’ commands were recorded (Fig. 1). Commands were provided in the patient's mother tongue, English or Spanish, via single-use headphones. For each patient, we recorded three blocks in which the patient was asked to move the right hand and three blocks in which the patient was asked to move the left hand (recordings of right-hand and left-hand blocks were alternated). Up to eight recordings were obtained from individual patients depending on availability of the patient. The Benjamini-Hochberg procedure for false discovery rate (FDR) correction was applied to address the problem of a varying number of assessments.34 The proportion of false positive results was bound at 5% when multiple recordings were obtained on a given patient. Data preparation included clipping of each five 2-s long EEG segments following command presentation and calculation of power spectral density in predefined frequency spectra [delta (1 to 3 Hz), theta (4 to 7 Hz), alpha (8 to 13 Hz), and beta (14 to 30 Hz)] for each recording electrode. These EEG features were analysed using a support vector machine (SVM) learning algorithm to determine if the EEG recording following a ‘keep …’ compared to a ‘stop …’ command systematically differed. The performance of the SVM classification using a linear kernel was determined based on the area under the receiver operating characteristic curve (AUC). A one-tailed permutation test was performed (500 times randomly shuffled labels) to determine the significance of the AUC (AUC > 0.5 after FDR correction for multiple recordings in a given patient).19 EEG analyses were performed using MNE-Python and Scikit-learn.35,36
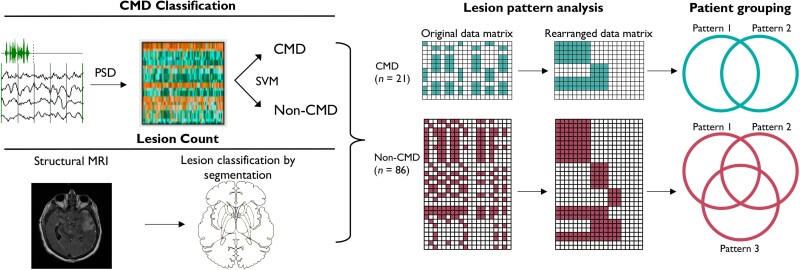
Figure 1.
CMD classification and lesion pattern analysis. Patients were assessed for cognitive motor dissociation (CMD) using EEG together with a motor command protocol. Retrospectively these patients were screened for having MRI with both diffusion weighted imaging (DWI) and fluid attenuated inversion recovery (FLAIR) sequences performed during their hospitalizations. Manual segmentation was conducted looking at the presence of any structural injury to the regions of interest based on signal change seen on T2-FLAIR alone as well as T2-FLAIR or DWI. Lesion pattern analysis was performed and identified two patterns of lesions unique to CMD patients and three patterns unique to non-CMD patients. Patients were then grouped based on lesion patterns; three and six groups were identified among CMD patients and non-CMD patients, respectively. PSD = power spectral density; SVM = support vector machine.
Resting EEG
EEG clips recorded following the ‘stop …’ command underwent additional analyses. In patients with CMD the first EEG that detected the CMD state closest to the MRI and in non-CMD patients, EEGs closest to the MRI acquisition underwent additional analyses. Raw EEG was visually inspected by a single investigator (J.C.) that was blinded to clinical, imaging, CMD status, and outcomes data and blocks rejected as artefact if electromyogenic, eye-blink, or electrical interference artefact were present.19,31,37 There was no prespecified rest period required between the behavioural assessments and the active EEG task.
Power spectral density
Power spectral density (PSD) plots were generated for each recording electrode by averaging spectra for each 3-s epoch using the multitaper method (Chronux toolbox, five tapers, yielding a frequency resolution of 2 Hz and estimates spaced 1/3 Hz apart).22,31 We then averaged these spectra within each rest block. PSD plots were classified by a single, blinded investigator (J.C.) applying the ABCD level classification based on spectral peaks within predefined frequency bands: ‘A’ with no peaks above delta (<4 Hz) range (indicating complete thalamocortical disruption), ‘B’ with only a theta (4–8 Hz) peak (indicating severe thalamocortical disruption), ‘C’ with both theta (4–8 Hz) and beta (13–24 Hz) peaks (indicating moderate thalamocortical disruption), and ‘D’ if both alpha (8–13 Hz) and beta (13–24 Hz) peaks were present (indicating normal thalamocortical function).22,31,38 The most favourable category (D > C > B > A) was assigned for spectra that met multiple requirements. As the degree of thalamocortical disconnection of other PSD spectra is less certain, plots were scored separately that did not fit these pre-specified criteria: ‘Y’ if there were only alpha peaks (8–13 Hz) and ‘Z’ if there were both alpha (8–13 Hz) and theta (4–8 Hz) peaks. All electrodes that were not scorable due to artefact were classified as ‘X’.
Functional connectivity
Coherence as a measure of functional connectivity was calculated between all 19 electrodes for all frequency bands using weighted pairwise phase consistency (WPPC).39 Coherence values range from 0 (completely incoherent) to 1 (perfectly coherent). In addition to individual pair-wise coherence measures, averaged coherence values were calculated for long- and short-range coherence.40 Neighbouring electrode pairs were excluded from the analysis. Short-range coherence was also averaged and reported by brain region.
MRI paradigm
MRI scans were obtained on a 3 T MRI scanner (GE Signa HDx MRI scanner; HD23 8 software). FLAIR, T1-weighted and DWI sequences were obtained (Supplementary Table 1).
Categorization of lesions
The presence of structural injury was determined based on signal change seen on T2-FLAIR. Forty-four anatomical ROIs were predefined based on established neuroanatomical atlases and selected for consideration in this study based on prior neuroanatomical and imaging studies of CMD and DoC (Table 1, Fig. 1 and Supplementary Fig. 1).26,29,41–47 Manual segmentation was used to label these ROIs for each MRI scan using high resolution T1 and T2 sequences by two board-certified neurologists (E.F., J.M.C.), that were blinded to the patients’ clinical assessments, hospital course, CMD status, and outcomes. In cases of disagreements, a third neurologist (J.C.) was used to adjudicate.44,48 An additional segmentation was conducted to determine the presence of early, temporary or permanent signal change seen in these ROIs taking into account both, abnormalities seen on T2- FLAIR or DWI sequences.
Table 1.
Anatomical regions of interest that were assessed for structural injury on brain MRI
| Region of interest | Number |
|---|---|
| Cerebellum (vermis, right, left) | 1,2,3 |
| Pons | |
| Anterior (central, right, left) | 4,5,6 |
| Tegmentum (central, right, left) | 7,8,9 |
| Midbrain (central, right, left) | 10,11,12 |
| Thalamus (right, left) | 13,14 |
| Anterior (right, left) | 15,16 |
| Lateral (right, left) | 17,18 |
| Medial (right, left) | 19,20 |
| Posterior (right, left) | 21,22 |
| Globus pallidus (right, left) | 23,24 |
| Putamen (right, left) | 25,26 |
| Caudate (right, left) | 27,28 |
| Internal capsule | |
| Anterior (right, left) | 29,30 |
| Posterior (right, left) | 31,32 |
| Insula (right, left) | 33,34 |
| Central hypothalamus | 35 |
| Central basal forebrain | 36 |
| Cortex | |
| Frontal (right, left) | 37,38 |
| Parietal (right, left) | 39,40 |
| Temporal (right, left) | 41,42 |
| Occipital (right, left) | 43,44 |
Patient outcomes
Command scores were obtained daily.19 The following neurological findings were recorded: no response, opening eyes to stimulation and/or eyes attending to stimulation, and following simple or complex commands.49 For purposes of analyses, assessments for command following were dichotomized into being able to or not being able to follow at least simple commands at any point in time during the hospitalization after the first CMD assessment.
Functional outcomes were assessed with the Glasgow Outcome Scale-Extended (GOS-E; ranging from 1 to 8, with higher levels corresponding to better outcomes) and were dichotomized at a GOS-E level of 4, a level that corresponds to the ability to be left up to 8 h without assistance.50 Outcomes were obtained at 12 months after injury via structured telephone interview with the patient or the caregiver if the patient was not able to be interviewed by phone. Patients, caregivers and the interviewers performing the outcome assessment were blinded to the results of the MRI lesion mapping and CMD assessment. CMD status was not provided to the treatment team and did not play a role in clinical decision making.
Patterns of brain lesions in CMD and non-CMD
The latent block model (LBM) approach was used to identify lesion patterns shared between groups of patients with and without CMD.51 All assessed brain regions were listed on the axes of a binary matrix. The LBM was applied to the binary lesion matrix , where when there was a lesion found in the jth region of the ith patient, 0 if not. The objective of the LBM algorithm was to find non-overlapping and exclusive homogeneous blocks by estimating the mixture model jointly on rows and columns of the data matrix. Then the modified Bayesian Information Criterion (BIC) was used for model selection, which was specially developed for the application to LBM.52 The best model was selected according to the maximal BIC, which controls overfitting by considering the goodness fit on data and the complexity of the model at the same time. In addition, due to the relatively small sample sizes, median Jaccard indices were used to evaluate the stability of the grouping of lesion patterns on the leave-one-out data.53 We applied identical procedures to the CMD and non-CMD data matrices.
Patient groupings based on shared lesion patterns
As groups of patients may share more than one of the identified lesion patterns, CMD and non-CMD patient groups were identified that shared either one or more of the identified lesion patterns.
Outcomes
To explore if any of the identified groups of CMD and non-CMD patients with shared lesion patterns had different outcomes, we explored their relationship to recovery of command following during the hospitalization, time to functional recovery by 12 months after injury, and time to death. Associations with command following were explored by logistic regression analysis. Association of lesion patterns with time to death and time to functional recovery defined as a GOS-E of 4 or above were analysed each using survival statistics. In models analysing time to functional recovery, dead patients were considered as censored and for the time to death models recovered patients were censored at 12 months.
Resting EEG
PSD plots were analysed using the ABCD classification as an ordinal scale (‘A’ worst and ‘D’ best) and by dichotomizing the ABCD scale based on the presence of a ‘D’ category. Comparisons between CMD and non-CMD patients were performed on an electrode-by-electrode level as well as determining the ‘best’ score across all electrodes. Additionally, we calculated the ‘ABCD index’ across all electrodes for each patient by averaging ABCD values across all electrodes (A = 1, B = 2, C = 3, D = 4; X, Y, Z = disregarded). We applied a two-sided Wilcoxon signed-rank test to compare ABCD indexes between patients with and without CMD. Other metrics were tested by Fisher's exact test. Significant differences between coherence measures were tested using a two-sided Wilcoxon signed-rank test, corresponding odds ratios (OR) and 95% confidence intervals (CI) were obtained via ordinal regression and logistic regression.
Statistical analysis
Categorical variables are presented as counts (percentages), and continuous variables presented as means (standard deviation, SD) or medians (interquartile range, IQR), as appropriate. Univariate associations between variables and outcomes were assessed with a Wilcoxon rank-sum test for quantitative variables or chi-squared test for qualitative variables. Statistical analyses were performed with R (version 4.0.3) statistical software. Logistic regression analyses were performed to evaluate for associations of cortical versus non-cortical lesion patterns with aetiology of injury.
Results
Patient cohort
Of 162 patients screened for CMD, 107 (66%) fulfilled our inclusion criteria, 55 patients (34%) were excluded as they did not receive MRI (n = 52), the relevant MRI sequences were motion degraded (n = 1) or the relevant MRI sequences were not available (n = 2). Comparing enrolled to excluded patients did not reveal differences in age, sex, race, primary language, GCS upon admission to the hospital, and distribution of primary admission diagnoses (Supplementary Table 2). Mean age of included patients was 62 (IQR 53,71) and 44% (n = 47) were female. The most common admission diagnosis was intracerebral haemorrhage (ICH, n = 33, 31%), followed by cardiac arrest (CA, n = 22, 21%), subarachnoid haemorrhage (SAH, n = 16, 15%), and TBI (n = 15, 14%).
CMD detection
Twenty-one of 107 (20%) included patients were diagnosed with CMD on median post-injury Day 7 (IQR 4, 13). Patients with CMD were similar to those without regarding age, primary admission diagnoses, sex, race, primary language, GCS on admission, time elapse between injury onset and MRI acquisition (Table 2).
Table 2.
Baseline characteristics of CMD patients and non-CMD patients
| CMD (n = 21) |
Non-CMD (n = 86) |
Odds ratio (95% CI) |
|
|---|---|---|---|
| Demographics | |||
| Age, median | 60 (50, 71) | 63 (54, 71) | 1.5 (0.6–3.8) |
| Sex, female | 10 (48) | 37 (43) | 1.2 (0.6–3.1) |
| Race | 0.9 (0.1–9.1) | ||
| White | 14 (67) | 49 (57) | |
| Black or African American | 6 (29) | 28 (33) | |
| Asian | 1 (5) | 4 (5) | |
| More than one race, or unknown or not reported | 0 (0) | 5 (6) | |
| Language | 6.0 (0.9–263.3) | ||
| English | 20 (95) | 66 (77) | |
| Spanish | 1 (5) | 20 (23) | |
| Admission findings | |||
| GCS on admission | 5 (4, 7) | 5 (3, 8) | 0.8 (0.3–2.0) |
| Aetiology | 1.7 (0.4–7.5) | ||
| Intracerebral haemorrhage | 7 (33) | 26 (30) | |
| Cardiac arrest | 3 (14) | 19 (22) | |
| Subarachnoid haemorrhage | 5 (24) | 11 (13) | |
| Traumatic brain injury | 4 (19) | 11 (13) | |
| Other | 2 (10) | 19 (22) | |
| Hospital course | |||
| Time to MRI (days) | 5 (2, 12) | 6 (3, 14) | 1.5 (0.6–3.8) |
| Time to EEG (days) | 3 (2, 4) | 4 (2, 9) | 2.3 (0.8–6.0) |
| Time to CMD diagnosis | 7 (4, 13) | NA | |
| Outcomes | |||
| Death at discharge | 2 (10) | 6 (7) | 1.4 (0.2–6.7) |
| GOS-E ≥ 4 by 12 months | 7(33) | 9 (10) | 4.3 (1.3–13.5) |
Table shows the median (interquartile range, IQR) except for aetiology, sex, race and language, where percentage is reported. Odds ratios for the categorical variables were computed using Fisher's exact test. Data for continuous and ordinal variable were split according to the median. CI = confidence interval; CMD = cognitive motor dissociation; GCS = Glasgow coma scale; GOS-E = Glasgow Outcome Scale-Extended; NA = not applicable.
Structural brain lesions on MRI
MRIs were obtained on median Day 6 (IQR 2, 14) after injury onset. Structural brain lesions on T2-FLAIR were most often identified in the frontal (right frontal n = 57 patients, 38% of the cohort; left frontal n = 48, 45%), parietal (right parietal n = 39, 36%; left parietal 33, 31%), temporal (right temporal n = 38, 36%; left temporal n = 29; 27%), and occipital cortex (right occipital n = 37, 35%; left occipital n = 35, 33%, Supplementary Table 3). In patients with CMD versus non-CMD, lesions were less frequently found in the left midbrain (0% versus 17%, P = 0.04) and left posterior thalamus (10% versus 31%, P = 0.04), and more frequently in the right posterior internal capsule (29% versus 9%, P = 0.02). One patient with CMD and seven patients without CMD were found to have no FLAIR or DWI lesions on MRI.
Patterns of brain lesions in CMD and non-CMD
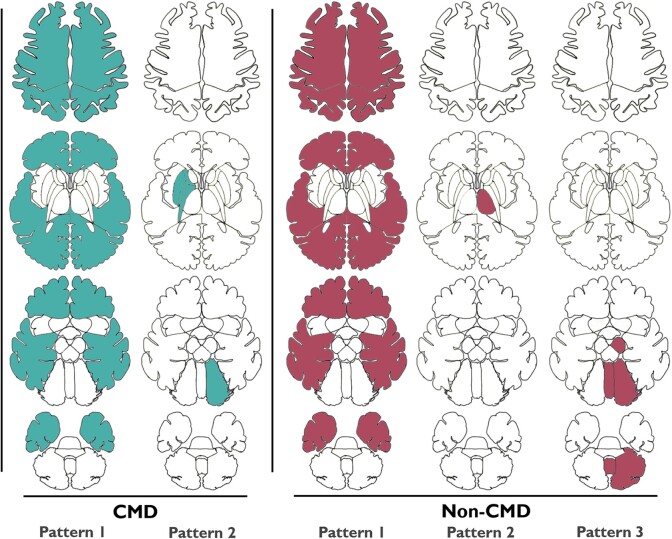
Modelling identified five distinct lesion patterns based on FLAIR sequences, two amongst CMD and three amongst non-CMD patients (Fig. 2). CMD Pattern 1 consisted of diffuse bilateral cortical injury (CMD Pattern 1: bilateral cortical) and CMD Pattern 2 had right-sided injury to the globus pallidus, putamen and anterior and posterior internal capsule, as well as left-sided cerebellar hemispheric injury (CMD Pattern 2: right anterior forebrain mesocircuit; Fig. 3 and Supplementary Table 4). Among non-CMD patients, non-CMD Pattern 1 consisted of diffuse bilateral cortical injury (non-CMD Pattern 1: bilateral cortical), non-CMD Pattern 2 had injury of the left thalamus including the anterior, posterior, medial and lateral portion (non-CMD Pattern 2: left thalamic), and non-CMD Pattern 3 had injury of the left midbrain, left cerebellum and vermis (non-CMD Pattern 3: brainstem-posterior fossa; Figs 2 and 4 and Supplementary Table 4). Analysing imaging data for FLAIR or DWI lesions did not change our groupings (Supplementary Figs 2 and 3).
Figure 2.
Anatomical distribution of lesion patterns for CMD and non-CMD patients. Axial cross sections of contributing lesions on FLAIR MRI in regions of interest in the five patterns identified by the biclustering analysis. CMD Pattern 1: bilateral cortical lesions. CMD Pattern 2: mesocircuit lesions. Non-CMD Pattern 1: bilateral cortical lesions. Non- CMD Pattern 2: left thalamic lesions. Non-CMD Pattern 3: brainstem lesions. CMD = cognitive motor dissociation.
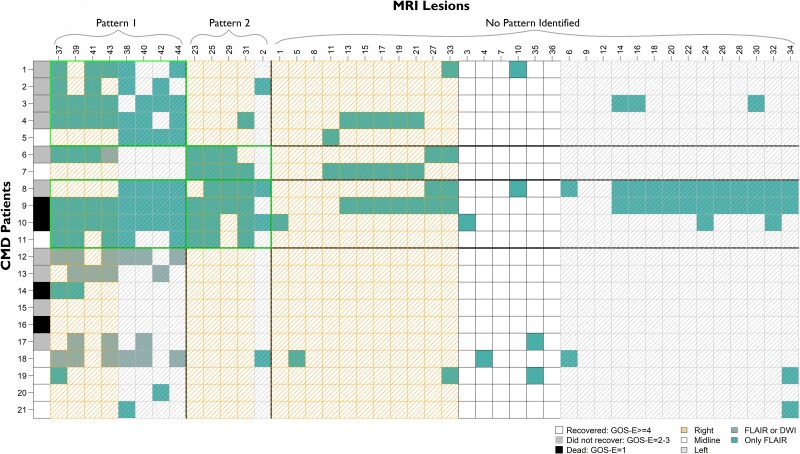
Figure 3.
Lesion patterns and patient groupings with outcomes in CMD patients. Three unique groups of CMD patients were identified based on CMD Patterns 1 and 2. Rows represent individual patients. Each column represents region of interest based on lesion number assigned (see Fig. 1). The right-most column shows outcome of each patients based on GOS-E at 12 months. CMD = cognitive motor dissociation; DWI = diffusion weighted imaging; FLAIR = fluid attenuated inversion recovery; GOS-E = Glasgow Outcome Scale-Extended.
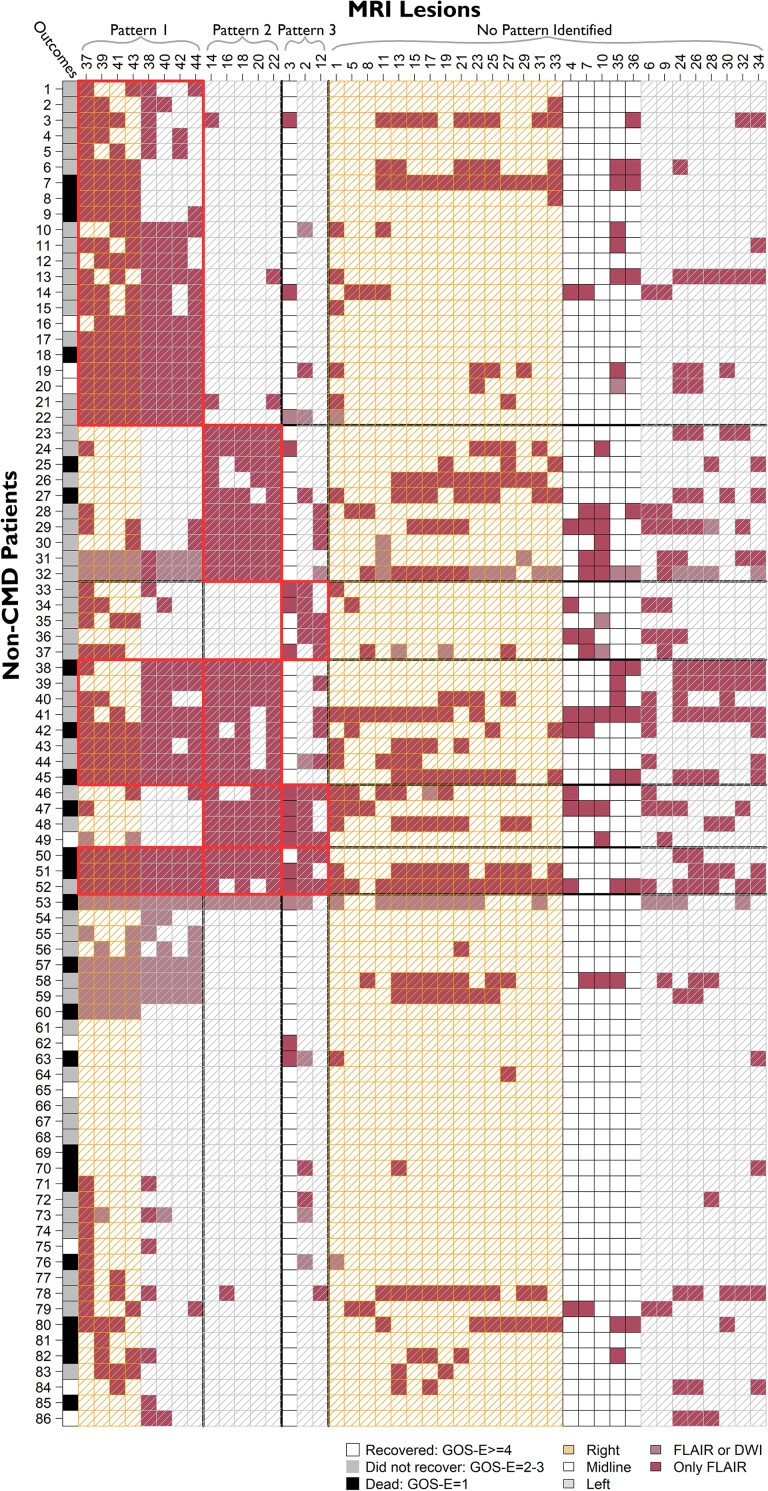
Figure 4.
Lesion patterns and patient groupings with outcomes in non-CMD patients. Six unique groups of non-CMD patients were identified based on non-CMD Patterns 1–3. Rows represent individual patients. Each column represents region of interest based on lesion number assigned (see Fig. 1). The right-most column shows outcome of each patients based on GOS-E at 12 months. CMD = cognitive motor dissociation; DWI = diffusion weighted imaging; FLAIR = fluid attenuated inversion recovery; GOS-E = Glasgow Outcome Scale-Extended.
Patient grouping based on shared lesion patterns
Three unique groups of CMD patients were identified: CMD Group 1 had bilateral cortical lesions of CMD Pattern 1 (n = 5), CMD Group 2 had lesions in the right anterior forebrain mesocircuit of CMD Pattern 2 (n = 2), and CMD Group 3 had both patterns of injury (n = 4). Ten patients (48%) had a pattern of lesions that did not fit in any of the identified groups (Fig. 3). Among non-CMD patients six unique groups were identified based on shared lesion patterns: non-CMD Group 1 only with bilateral cortical lesions of the non-CMD Pattern 1 (n = 22), non-CMD Group 2 only with left thalamic lesions of non-CMD Pattern 2 (n = 15), and non-CMD Group 3 only with brainstem-posterior fossa lesions of non-CMD Pattern 3 (n = 10). Fifteen patients had lesions from more than one of the non-CMD patterns; eight with non-CMD Patterns 1 and 2 (non-CMD Group 4), four with non-CMD Patterns 2 and 3 (non-CMD Group 5), three patients with non-CMD Patterns 1, 2 and 3 (non-CMD Group 5). Thirty-four patients (40%) had a pattern of lesions that did not fit in any of the identified groups (Fig. 4).
Aetiology of injury based on lesion pattern
Injury aetiologies were distributed across all CMD and non-CMD injury patterns (Supplementary Table 5). Patients with ICH were over-represented in non-CMD Pattern 2 (six patients with ICH of eight total with non-CMD Pattern 2) and TBI patients were more common amongst cortical (CMD Pattern 1 and non-CMD Pattern 1) than non-cortical lesion patterns (CMD Pattern 2 and non-CMD Patterns 2 or 3).
Patient outcomes
Forty-four patients (41% of the entire cohort, 10 CMD and 34 non-CMD) followed commands prior to hospital discharge and at 1 year after the injury 52 (49%) patients were dead and 16 (15%) had recovered to a GOS-E of 4 or better. Patients with CMD were more likely to have recovered to a GOS-E level of ≥4 by 12 months than patients without CMD (OR 4.3, 95% CI 1.3–13.5). Rates of withdrawal of care did not differ between patients with and without CMD (OR 1.1, 95% CI 0.3–3.2).
Power spectral density plots
For 78 patients (14 CMD versus 64 non-CMD) at least one PSD plot fit the ABCD classification and 24 (31%; 0 CMD versus 24 non-CMD) were classified as the highest score of ‘A’, 31 (40%; one CMD versus 30 non-CMD) of ‘B’, 16 (20%; eight CMD versus eight non-CMD) of ‘C’, and seven (9%; five CMD versus two non-CMD) of ‘D’. Twenty-six patients had plots that were all classified as unscoreable due to artefact, three patients that did not fit the ABCD criteria as there were only alpha and theta peaks were classified as ‘Y’ or ‘Z’, or both (one with both, one each with ‘Y’ or ‘Z’ only).
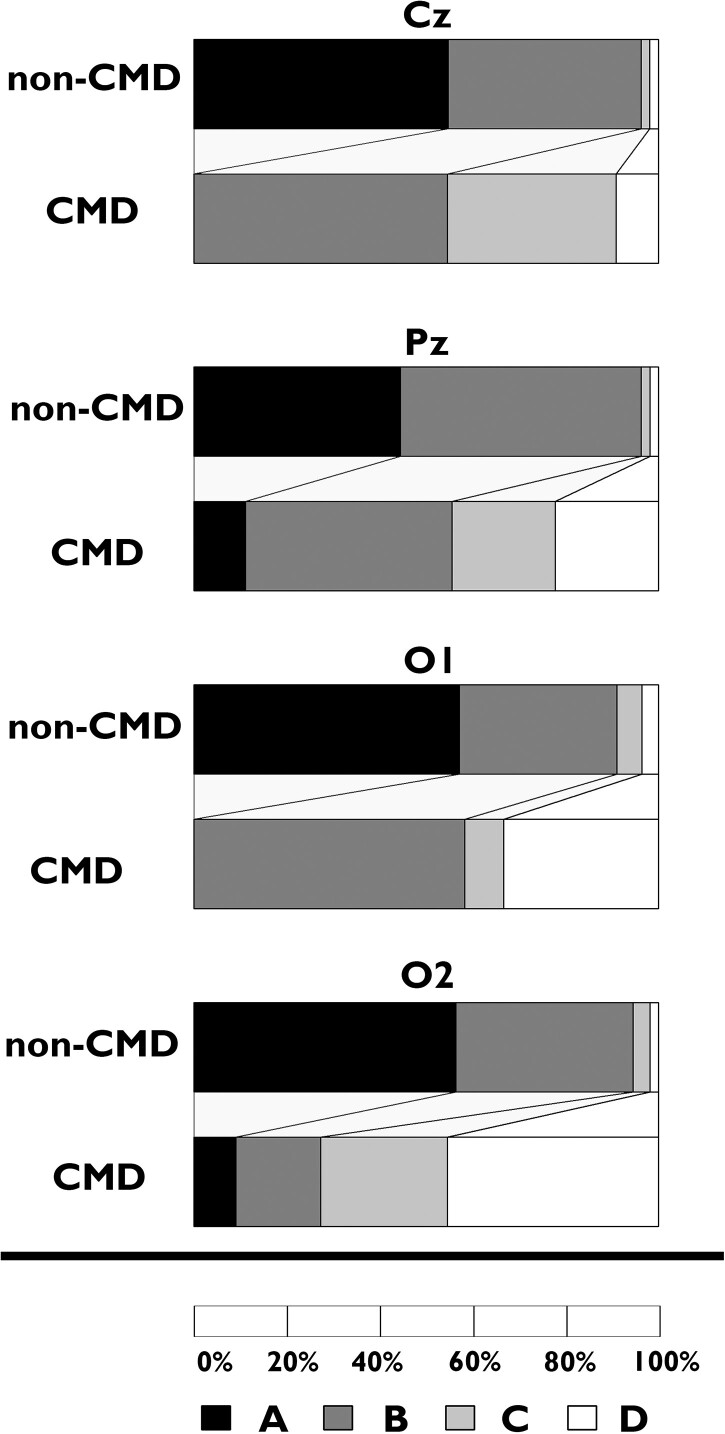
Higher ABCD scores were seen for patients with than those without CMD across most electrodes, particularly for those without bilateral cortical lesions (Fig. 5, Supplementary Table 6 and Supplementary Fig. 4). The number of patients with a PSD plot categorized as D supporting no thalamocortical dysfunction was higher in those with compared to those without CMD (44% versus 2%, OR 28.1, 95% CI 2.3–1592.8). The ABCD index was 2.8 (IQR 2.1, 3.1) versus 1.5 (IQR 1.0, 2.0) for patients with and without CMD, respectively (OR 8.4, 95% CI 2.6–43.7). In patients with bilateral cortical lesions, less difference in ABCD scores was seen for all electrodes. EEG electrodes with a D score as the highest value were seen in 20% of CMD and 4% of non-CMD patients. The ABCD index was 2.1 (IQR 2.1, 2.3) versus 1.3 (IQR 1.0, 2.0) for patients with and without CMD, respectively (OR 2.7, 95% CI 0.9–10.2).
Figure 5.
ABCD classification of power spectral density plots in patients without bilateral cortical lesions. Cz, Pz, O1 and O2 refer to representative EEG electrode positions.
Functional connectivity: weighted pairwise phase consistency
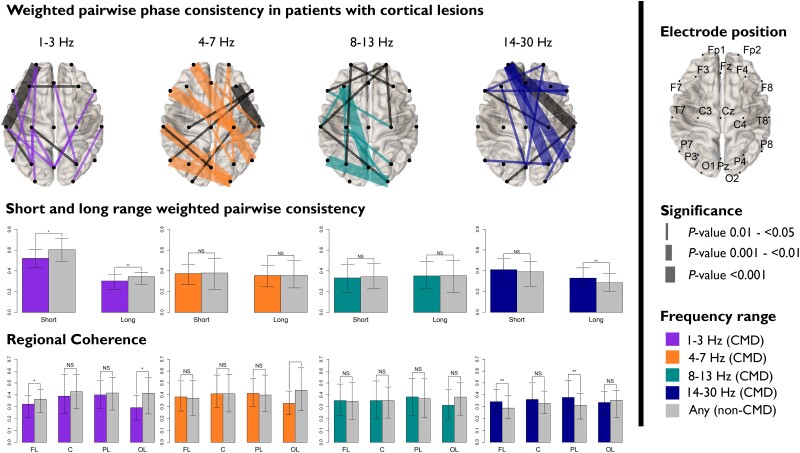
Among patients with bilateral cortical lesions, short-range [median 0.5 (IQR 0.4–0.6) with CMD versus 0.6 (0.5–0.7) without CMD; OR 0.7 (95% CI 0.5–1.0)] and long-range WPPC [0.3 (0.2–0.4) versus 0.4 (0.3–0.4), OR 0.8 (0.7–0.9)] were decreased in the delta frequency spectrum (1–3 Hz) for CMD when compared to non-CMD patients (Fig. 6 and Supplementary Table 7). This difference in WPPC was particularly prominent in the frontal [0.3 (0.2–0.4) versus 0.4 (0.3–0.4), OR 0.8 (0.7–1.0)] and occipital regions delta frequency spectrum [0.3 (0.2–0.4) versus 0.4 (0.2–0.6), OR 0.6 (0.4–0.9)] and in the and occipital region in the theta frequency (4–7 Hz) spectrum [0.3 (0.2–0.4) versus 0.4 (0.2–0.6), OR 0.6 (0.4–0.9)]. Increased beta frequency (14–30 Hz) long-range WPPC was seen for CMD patients [0.3 (0.2–0.4) versus 0.3 (0.2–0.4), OR 1.2 (1.0–1.5)], particularly in the frontal [0.3 (0.3–0.5) versus 0.3 (0.3–0.4), OR 1.3 (1.1–1.6)] and parietal lobes [0.4 (0.3–0.5) versus 0.3 (0.2–0.4), OR 1.4 (1.0–1.8)]. No differences in WPPC were seen in patients without cortical lesions (Supplementary Fig. 5).
Figure 6.
Weighted pairwise phase consistency (WPPC) differences between CMD and non-CMD in patients with bilateral cortical lesions. Top row: spatial plots of individual electrode pairs by frequency ranges. Middle row: median short- and long-range WPPC; and bottom row: regional WPCC for patients with and without CMD. C = central; CMD = cognitive motor dissociation; FL = frontal lobe; NS = not significant; OL = occipital lobe; PL = parietal lobe.
Discussion
Underlying mechanisms for the dissociation between volitional motor planning and motor output also referred to as CMD or covert consciousness are poorly understood. Prior studies failed to identify a single unifying structural lesion common to patients with or without CMD.19 Abnormalities in thalamocortical projections and global structural brain connectivity were increased in CMD, but systematic large-scale studies that identify a responsible lesion or patterns of lesions are missing.26,27 Applying a lesion pattern analysis approach, we identified two patterns common among CMD and three among non-CMD patients. These structural lesion patterns, together with an analysis of large-scale functional connectivity (i.e. WPPC) confirm cardinal requirements for CMD, including preserved arousal, speech comprehension, and integration with the motor output in order to act out motor commands. Lesions in patients with CMD suggest failure to integrate comprehended motor commands either at the level of the cortex (e.g. bilateral cortical lesions) or the basal ganglia (e.g. putamen and globus pallidus) and contralateral cerebellum. Initiating motor commands following successful decoding of a verbal command in the temporal lobe requires large scale integration of posterior parietal, somatosensory, premotor and motor cortices, which may be impaired in patients with bilateral cortical injury.4,54 Further downstream integration and crucial adjustments of movements occur at the level of the basal ganglia (i.e. putamen and globus pallidus) and the contralateral cerebellum.5 These basal ganglia structures have been implicated in reversible DoC as part of the anterior forebrain mesocircuit.42,55,56 Lesions in these brain regions, involved in circuit level integration of motor commands with motor output, provide plausible candidates to approach a mechanistic understanding of CMD that may present targets for interventions.28
Arousal is a prerequisite for consciousness and not surprisingly lesions within classic midbrain arousal pathways were identified in our study for a group of non-CMD patients. Midbrain lesions were only seen in non-CMD patients and often identified together with widespread, bilateral cerebellar injury. Large cerebellar strokes or bleeds frequently functionally impair brainstem arousal centres causing mass effect on these pathways projecting to the cortex.9,57 Brainstem lesions in general were very rare in CMD patients and if present only limited (Fig. 3). Unilateral midbrain lesions alone were seen in a few of our non-CMD patients and these do not necessarily cause unconsciousness.41 However, when combined with lesions in thalamic and extra-thalamic pathways of the ascending reticular activating system (ARAS), as in our study (Fig. 4), complex arousal networks are affected and consciousness can be impaired.57–59 Further evidence for a functional link between the midbrain and thalamus has been provided by anaesthesia-induced studies of impaired consciousness.57,60 Increase in brainstem-thalamic connectivity during recovery of consciousness after traumatic coma has been demonstrated using diffusion tensor imaging analysis.61
At the cortical level, impaired arousal may present as downregulation of neuronal firing rates also known as disfacilitation and can result from lack of excitatory input, such as from lesions described above, or a result of direct structural loss as seen in diffuse, bilateral neocortical injuries.62–65 This latter phenomenon may underlie non-CMD patients with widespread bilateral injury patterns seen in our study further corroborated by our electrophysiological resting state data. When compared to CMD patients with widespread bilateral injuries, these patients had broadly decreased neuronal activity seen in our PSD analysis. CMD patients with bilateral cortical lesions were found to have increased PSD, particularly in higher frequency ranges when compared to unresponsive patients without CMD. This may suggest intact arousal and is likely in part a reflection of the underlying pathophysiology, as previously described.31
CMD detection uses verbal commands making comprehension of a verbal motor command a crucial prerequisite to be classified as CMD-positive. Left thalamic lesions were shared amongst a group of non-CMD patients and were only in slightly more than half of the cases associated with right thalamic injury (Fig. 4). Particularly, unilateral dominant hemispheric thalamic injury may suggest impaired speech comprehension, which may present a challenge for existing CMD paradigms that are largely built on auditory presentation of motor imagery or motor commands.66–69 However, the thalamus is also a crucial relay in motor planning and lesions herein may also impair preparation of a movement.70 Stimulation of central thalamic nuclei via electric deep brain stimulation has been shown to improve function in patients with severely impaired consciousness following trauma and anaesthesia-induced unconsciousness.71,72 Low intensity focused ultrasound targeting the thalamus has been associated with faster recovery from anaesthesia and improved behavioural responsiveness in three DoC patients.73,74 Additionally, large-scale cortical network activation is required for processing language-related tasks, such as motor commands.54 Cortical lesions that could interfere with this large-scale integration of commands with motor planning were frequently identified in non-CMD patients with unilateral thalamic injury, when compared to CMD patients.
If patients with CMD are conscious and cognition at the level of wilful modulation of brain activity is present then the above neurological functions, i.e. arousal and command comprehension, need to be intact. The dissociation between cognition and behaviour has to lie downstream resulting in a failure to act out the motor command. In clinical assessments of consciousness, commands demanding movements are utilized which, in the neurologically intact, activate supplementary motor areas as well as the primary motor cortex.70 This coordinated motor planning leading to activation of the respective areas within the primary motor cortex could plausibly be impaired by widespread bilateral cortical lesions. In patients that have intact arousal and speech comprehension, this may lead to a dissociation between cognition (i.e. motor command comprehension) and motor expression (i.e. demonstrating movement consistent with command following). Despite an ongoing debate about the provenance of anterior versus posterior brain regions for consciousness, large scale integration of the information is clearly a prerequisite for consciousness.75 The electrophysiology data on resting EEG collected in our study support this notion. A broad frequency shift, comparing conscious with unconscious patients, is seen acutely and chronically following brain injury.49,76,77 Conceptually, these data are in line with predictable changes in functional thalamocortical network integrity, which are expressed in degrees of impaired consciousness along the ABCD framework, seeing increased lower frequencies with impairment and increased higher frequencies with intact or recovered consciousness.37
We found that patients with CMD, when compared to non-CMD patients, had better functional connectivity across large areas of the brain as reflected in higher coherence metrics within a number of different frequency spectra. This is in line with findings suggesting possible functional connectivity metrics in CMD patients that are closer to healthy volunteers than behaviourally unresponsive patients without CMD.78 Prior studies demonstrated that impaired functional connectivity may be measured by electrophysiological approaches such as resting state EEG (weighted symbolic mutual information or coherence).76 These findings integrate well with analyses of structural brain networks that revealed more complex connectomes in CMD patients, suggesting that focal motor disconnection syndromes may be superimposed on a complex architecture of global brain connectivity in this state.27
Frontoparietal networks that are comprised of the default mode network (DMN) and the executive control network have been reliably identified in disorders and recovery of consciousness.79,80 The DMN relates closely to the level of consciousness in brain injured patients and mediates internal awareness or self-related processes.80–82 Resting state networks are incompletely investigated in CMD patients but earlier work had shown a relationship between DMN re-emergence and recovery of consciousness.83,84 Most recently, a study including CMD patients with acute brain injury challenged this notion as CMD was diagnosed without an intact DMN.61
Lesions in the anterior forebrain mesocircuit have increasingly been implicated in patients with DoC classified based on verbal motor imagery or motor commands. This feedback loop focuses on the role of central thalamic neurons and their frontostriatal connections that modulate input to broad cortical areas and receive inputs from lower lying brainstem regions.42,55,56 Lesions in the striatum result in loss of inhibitory projections from medium spiny neurons to the globus pallidus internus, which then inhibits central thalamic nuclei.85 Cardinal structures of the anterior forebrain mesocircuit, such as the globus pallidus and putamen, were identified as shared lesions amongst CMD patients in the present study. Lesions in the globus pallidus and putamen integrate well with the concept of circuit-level dysfunction proposed within the anterior forebrain mesocircuit model. However, these structures also serve as relays in motor planning and execution. The striatum takes a central role of selecting the most appropriate motor programmes from the available choices in a given situation. Additional lesions in this group of patients map onto structures important in motor planning and execution (i.e. anterior and posterior internal capsule, and the contralateral cerebellar hemisphere). It remains unclear how exactly these lesions together would lead to CMD but investigating functional connectivity in subcortical structures is challenging by means of electrophysiology and will require advanced neuroimaging explorations.
Interestingly, subcortical lesions in the anterior forebrain mesocircuit (CMD Pattern 2) are all located on the right side implicating motor neglect as a possible phenomenon contributing to the CMD state.86 Left-sided lesions with or without motor neglect may not be detected as CMD as more commonly language comprehension would be expected to be impaired. While left-sided lesions typically present with contralateral neglect, right-sided lesions may result in bilateral lack of motoric activation. Specifically, right-sided subcortical lesions have been associated with neglect phenomena attributed to imbalanced hemispheric activation.87,88
Cerebellar lesions were identified in two of the lesion pattern models, one of the CMD and one of the non-CMD patients. The cerebellum has been increasingly implicated as a crucial hub for motor function that includes planning, acting, learning and adjustments.5,89 With a particular focus on predictions and corrections of motor actions, contralateral lesions in the cerebellum may contribute to the phenomenon of CMD if paired with anterior forebrain mesocircuit lesions as long as arousal and comprehension are preserved. In CMD patients with cerebellar lesions, midbrain injury was infrequent when compared to non-CMD patients with cerebellar lesions.
Outcomes
We confirm in the cohort studied here that patients with CMD were more likely to have recovered by 12 months after injury.8,19 Injury mechanisms were diverse and likely a major cause for differences in outcomes. Even though several isolated electrode pairs demonstrated significantly increased or decreased localized functional connectivity (i.e. alpha frequency WPPC), when we compared patients with and without CMD amongst those with (Supplementary Fig. 5) and without cortical lesions (Fig. 6), short- or long-range functional connectivity in the alpha frequency range did not differ in grouped analysis. This observation is interesting as prior studies linked CMD with better recovery of consciousness and function and several studies linked enriched alpha frequency connectivity with better outcomes in acute and chronic DoC.19 Further studies observed enriched alpha connectivity in both traumatic and non-traumatic patients with DoC.90–92
Patients have been hierarchically categorized based on careful clinical examination with certain states such as MCS+ when compared to coma, vegetative state/unresponsive wakefulness syndrome, and MCS− being associated with a higher chance of recovery.93,94 The label MCS has been discussed controversially as the content and quality of consciousness is uncertain, a critique that applies even more so to CMD.95 The term ‘cortically mediated state’ has been proposed as this more descriptively describes the phenomenon. Even though CMD has been linked to better outcomes and to a large extent the etymology of terms is a philosophical discussion, in this space labels may have future implications for goals of care and access to therapeutic interventions.8,19 Taking this into account, labels should be chosen with care and should be clearly distinguished from one another.
Generalizability
Patients included in this study represent an unselected cohort of unresponsive patients admitted to the neurological ICU but patients that quickly recovered consciousness, were unable to be monitored with EEG (i.e. surgical wounds preventing electrode placement) or undergo MRI scanning (i.e. cardiac pacemaker) and are therefore under-represented here. Fewer patients with isolated brainstem injury and with non-structural causes for coma were seen in our sample, which is attributable to our patient ascertainment. Taking these considerations into account, the assumption of generalizability to the broader population of acutely unconscious patients can be made as supported by the follow-up bias analysis. The lesion pattern analysis approach allowed us to identify not only groups of patients that share lesions in a specific brain region, but rather lesion patterns that may together better account for a specific behavioural state. One prior study correlated the frequency of brain lesions identified on MRI with subtle behavioural evidence of command-following detected only by the MBT-r.29 Structural injury in the left mesencephalon, right basal ganglia, right thalamus, right parietal cortex, and left frontal cortex were seen in patients with subtle behavioural evidence of command-following not detected by the gold standard approach, the CRS-R.30 As this approach is based on detecting subtle behavioural signs, it may identify dissociations between the gold standard behavioural assessment, the CRS-R, and this modified motor assessment but without the assistance of fMRI or EEG it cannot identify patients that have brain activation without any detectable behavioural evidence of command-following. It therefore does not get to the heart of the dissociation between brain activation without motor execution.
Limitations
This study has several limitations including that (i) structural imaging was not obtained at predefined time points following the injury; (ii) we were not able to quantify abnormalities in structural connectivity as DTI sequences were not routinely obtained; (iii) we did not assess functional connectivity by resting state MRI sequences; (iv) there was some variability in the CMD assessments (e.g. time from injury, number of assessments); (v) ‘resting state EEG’ clips were taken from EEG obtained following the ‘stop opening …’ commands and not following true rest (i.e. 5 min of eyes open), and additional post hoc artefact cleaning was applied31,37; and (vi) for practical reasons, a limited catalogue of verbal prompts was used to test command following using both, the CSR-R protocol for behavioural classification and EEG responses for CMD detection. It is possible that a larger catalogue with varying degrees of difficulty could have generated additional response information but in the critical care context this has to be weighed against the limited time of access to the patient and fatigability of the patient, particularly when repeated assessments are desired and builds on prior studies.8,19,20,30 The CMD protocol requires repetitions to generate a large enough dataset to allow CMD detection, which would be particularly challenging with a catalogue of many different commands. To account for varying numbers of CMD assessments (range 1–8 assessments), FDR correction was applied to minimize the rate of false positive detections.8,19,20 However, following acute brain injury behavioural states may not be as static as in the chronic brain injury context. Therefore, overly rigorous FDR correction may not only decrease the rate of false positives but also increase the rate of false negatives. Though structural imaging was not obtained at predefined time points, using lesion pattern analysis, neither the lesion patterns nor the patient groupings changed when the analysis was done on FLAIR lesions only versus FLAIR and DWI lesions. This suggests that timing of imaging did not affect results of lesion pattern analysis to a large degree. Of the patients included in our analysis 48% (n = 10) CMD and 33% (n = 28) non-CMD did not fit into any of the groups identified by the lesion pattern analysis. The lesion pattern analysis was conducted on a sample of 21 CMD and 86 non-CMD patients. It is possible that with a large sample size, additional unique patterns of injury would be identified, and these patients would fall into the newly identified groups, and that these newly identified patterns of injury would be associated with varying prognoses. The LBM approach was employed to identify frequently shared lesion patterns amongst CMD and non-CMD patients, as in clinical reality few patients have identical lesions. Additionally, with a larger sample size, it is possible that a link between lesion pattern and aetiology would emerge, supporting that CMD is related to aetiology rather than pattern of injury. Last, the study confirms known limitations of verbal command based CMD testing in patients with possible aphasia.
Conclusion
The identified lesions and connectivity patterns support the notion that focal lesions at the anterior forebrain mesocircuit level with preserved thalamocortical projections as well as bilateral focal cortical lesions with preserved functional connectivity may be seen in patients with CMD. Not surprisingly, lesions in brain regions supporting arousal and speech comprehension may be encountered in some patients without CMD. These insights allow more accurate phenotyping of CMD patients that may in the future allow targeted interventional trials with a higher success rate and more accurate prognostication of the recovery trajectory.96–99
Supplementary Material
Acknowledgements
We thank the nurses, attendings, fellows and neurology residents of the Neuroscience ICU for their overall support of this project.
Contributor Information
Eva Franzova, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Qi Shen, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Kevin Doyle, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Justine M Chen, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Jennifer Egbebike, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Athina Vrosgou, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Jerina C Carmona, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Lauren Grobois, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Gregory A Heinonen, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Angela Velazquez, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Ian Jerome Gonzales, NewYork-Presbyterian Hospital, New York, NY, USA.
Satoshi Egawa, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Sachin Agarwal, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
David Roh, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Soojin Park, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
E Sander Connolly, Department of Neurological Surgery, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Jan Claassen, Department of Neurology, Columbia University Medical Center, NewYork-Presbyterian Hospital, New York, NY, USA.
Funding
Financial support and sponsorship: J.C. is supported by grant funding from the NIH R01 NS106014 and R03 NS112760, and the DANA Foundation.
Competing interests
The authors report no competing interests.
Data availability
Raw data were generated at New York Presbyterian Hospital—Columbia University. Derived data supporting the findings of this study are available from the corresponding author on request.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17:135–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kondziella D, Amiri M, Othman MH, et al. . Incidence and prevalence of coma in the UK and the USA. Brain Commun. 2022;4:fcac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996;77:45–52. [DOI] [PubMed] [Google Scholar]
- 4. Haar S, Donchin O. A revised computational neuroanatomy for motor control. J Cogn Neurosci. 2020;32:1823–1836. [DOI] [PubMed] [Google Scholar]
- 5. Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaidya AR, Pujara MS, Petrides M, Murray EA, Fellows LK. Lesion studies in contemporary neuroscience. Trends Cogn Sci. 2019;23:653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. [DOI] [PubMed] [Google Scholar]
- 8. Egbebike J, Shen Q, Doyle K, et al. . Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: A prospective observational cohort study. Lancet Neurol. 2022;21:704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Posner JB, Saper CB, Schiff ND, Claassen J. Plum and posner’s diagnosis and treatment of stupor and coma. Oxford University Press; 2019. [Google Scholar]
- 10. Kondziella D. Roald dahl and the complete locked-in syndrome: “cold dead body, living brain”. J Neurol Sci. 2017;379:276–278. [DOI] [PubMed] [Google Scholar]
- 11. Jeannerod M. Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. [DOI] [PubMed] [Google Scholar]
- 12. Jackson PL, Decety J. Motor cognition: A new paradigm to study self–other interactions. Curr Opin Neurobiol. 2004;14:259–263. [DOI] [PubMed] [Google Scholar]
- 13. Fins JJ, Schiff ND. Shades of gray: New insights into the vegetative state. Hastings Cent Rep. 2006;36:8. [DOI] [PubMed] [Google Scholar]
- 14. Thibaut A, Panda R, Annen J, et al. . Preservation of brain activity in unresponsive patients identifies. Ann Neurol. 2021;90:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giacino JT, Schnakers C, Rodriguez-Moreno D, Kalmar K, Schiff N, Hirsch J. Behavioral assessment in patients with disorders of consciousness: Gold standard or fool’s gold? Prog Brain Res. 2009;177:33–48. [DOI] [PubMed] [Google Scholar]
- 16. Bruno M-A, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J Neurol. 2011;258:1373–1384. [DOI] [PubMed] [Google Scholar]
- 17. Schnakers C, Bauer C, Formisano R, et al. . What names for covert awareness? A systematic review. Front Hum Neurosci. 2022;16:971315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015;72:1413–1415. [DOI] [PubMed] [Google Scholar]
- 19. Claassen J, Doyle K, Matory A, et al. . Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–2505. [DOI] [PubMed] [Google Scholar]
- 20. Edlow BL, Chatelle C, Spencer CA, et al. . Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140:2399–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:485–492. [DOI] [PubMed] [Google Scholar]
- 22. Forgacs PB, Frey HP, Velazquez A, et al. . Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol. 2017;4:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stender J, Gosseries O, Bruno M-A, et al. . Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet. 2014;384:514–522. [DOI] [PubMed] [Google Scholar]
- 24. Luppi AI, Cain J, Spindler LRB, et al. . Mechanisms underlying disorders of consciousness: Bridging gaps to move toward an integrated translational science. Neurocrit Care. 2021;35(Suppl 1):37–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snider SB, Edlow BL. MRI In disorders of consciousness. Curr Opin Neurol. 2020;33:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández-Espejo D, Rossit S, Owen AM. A thalamocortical mechanism for the absence of overt motor behavior in covertly aware patients. JAMA Neurol. 2015;72:1442–1450. [DOI] [PubMed] [Google Scholar]
- 27. Luppi AI, Craig MM, Coppola P, et al. . Preserved fractal character of structural brain networks is associated with covert consciousness after severe brain injury. NeuroImage Clin. 2021;30:102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thibaut A, Chatelle C, Vanhaudenhuyse A, et al. . Transcranial direct current stimulation unveils covert consciousness. Brain Stimul. 2018;11:642–644. [DOI] [PubMed] [Google Scholar]
- 29. Pincherle A, Jöhr J, Chatelle C, et al. . Motor behavior unmasks residual cognition in disorders of consciousness. Ann Neurol. 2019;85:443–447. [DOI] [PubMed] [Google Scholar]
- 30. Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: Measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 31. Curley WH, Forgacs PB, Voss HU, Conte MM, Schiff ND. Characterization of EEG signals revealing covert cognition in the injured brain. Brain. 2018;141:1404–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bekinschtein TA, Manes FF, Villarreal M, Owen AM, Della-Maggiore V. Functional imaging reveals movement preparatory activity in the vegetative state. Front Hum Neurosci. 2011;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldfine AM, Victor JD, Conte MM, Bardin JC, Schiff ND. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol. 2011;122:2157–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 35. Gramfort A. MEG And EEG data analysis with MNE-python. Front Neurosci. 2013;7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedregosa F, Varoquaux G, Gramfort A, et al. . Scikit-learn: Machine learning in pytho. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 37. Curley WH, Bodien YG, Zhou DW, et al. . Electrophysiological correlates of thalamocortical function in acute severe traumatic brain injury. Cortex. 2022;152:136–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alkhachroum A, Eliseyev A, Der-Nigoghossian CA, et al. . EEG To detect early recovery of consciousness in amantadine-treated acute brain injury patients. J Neurol Neurosurg Psychiatry. 2020;91:675–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinck M, van Wingerden M, Womelsdorf T, Fries P, Pennartz CMA. The pairwise phase consistency: A bias-free measure of rhythmic neuronal synchronization. Neuroimage. 2010;51:112–122. [DOI] [PubMed] [Google Scholar]
- 40. den Bakker H, Sidorov MS, Fan Z, et al. . Abnormal coherence and sleep composition in children with angelman syndrome: A retrospective EEG study. Mol Autism. 2018;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–1536. [DOI] [PubMed] [Google Scholar]
- 42. Schiff ND. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lutkenhoff ES, Chiang J, Tshibanda L, et al. . Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol. 2015;78:68–76. [DOI] [PubMed] [Google Scholar]
- 44. Rohaut B, Doyle KW, Reynolds AS, et al. . Deep structural brain lesions associated with consciousness impairment early after hemorrhagic stroke. Sci Rep. 2019;9:4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snider SB, Hsu J, Darby RR, et al. . Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum Brain Mapp. 2020;41:1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fischer DB, Boes AD, Demertzi A, et al. . A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li J, Curley WH, Guerin B, et al. . Mapping the subcortical connectivity of the human default mode network. Neuroimage. 2021;245:118758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Despotović I, Goossens B, Philips W. MRI Segmentation of the human brain: Challenges, methods, and applications. Comput Math Methods Med. 2015;2015:450341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Claassen J, Velazquez A, Meyers E, et al. . Bedside quantitative electroencephalography improves assessment of consciousness in comatose subarachnoid hemorrhage patients. Ann Neurol. 2016;80:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: Observations on the use of the Glasgow outcome scale. J Neurol Neurosurg Psychiatry. 1981;44:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Govaert G, Nadif M. Clustering with block mixture models. Pattern Recognit. 2003;36:463–473. [Google Scholar]
- 52. Keribin C, Brault V, Celeux G, Govaert G. Estimation and selection for the latent block model on categorical data. Stat Comput. 2015;25:1201–1216. [Google Scholar]
- 53. Tang M, Kaymaz Y, Logeman BL, et al. . Evaluating single-cell cluster stability using the jaccard similarity index. Bioinformatics. 2021;37:2212–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schiff ND, Rodriguez-Moreno D, Kamal A, et al. . fMRI reveals large-scale network activation in minimally conscious patients. Neurology. 2005;64:514–523. [DOI] [PubMed] [Google Scholar]
- 55. Fridman EA, Beattie BJ, Broft A, Laureys S, Schiff ND. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A. 2014;111:6473–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams ST, Conte MM, Goldfine AM, et al. . Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. Elife. 2013;2:e01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Edlow BL, Takahashi E, Wu O, et al. . Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Snider SB, Bodien YG, Bianciardi M, Brown EN, Wu O, Edlow BL. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93:e1281–e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edlow BL, Haynes RL, Takahashi E, et al. . Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fiset P, Paus T, Daloze T, et al. . Brain mechanisms of propofol-induced loss of consciousness in humans: A positron emission tomographic study. J Neurosci. 1999;19:5506–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Snider SB, Bodien YG, Frau-Pascual A, Bianciardi M, Foulkes AS, Edlow BL. Ascending arousal network connectivity during recovery from traumatic coma. NeuroImage Clin. 2020;28:102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Steriade M, Nunez A, Amzica F. A novel slow (<1Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Timofeev I. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. [DOI] [PubMed] [Google Scholar]
- 66. Schaller-Paule MA, Oeckel AM, Schüre J-R, et al. . Isolated thalamic stroke—Analysis of clinical characteristics and asymmetry of lesion distribution in a retrospective cohort study. Neurol Res Pract. 2021;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fritsch M, Krause T, Klostermann F, Villringer K, Ihrke M, Nolte CH. “Thalamic aphasia” after stroke is associated with left anterior lesion location. J Neurol. 2020;267:106–112. [DOI] [PubMed] [Google Scholar]
- 68. Alain C, Reinke K, McDonald KL, et al. . Left thalamo-cortical network implicated in successful speech separation and identification. Neuroimage. 2005;26:592–599. [DOI] [PubMed] [Google Scholar]
- 69. Klostermann F, Krugel LK, Ehlen F. Functional roles of the thalamus for language capacities. Front Syst Neurosci. 2013;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guo ZV, Inagaki HK, Daie K, Druckmann S, Gerfen CR, Svoboda K. Maintenance of persistent activity in a frontal thalamocortical loop. Nature. 2017;545:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schiff ND, Giacino JT, Kalmar K, et al. . Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. [DOI] [PubMed] [Google Scholar]
- 72. Schiff ND. Central lateral thalamic nucleus stimulation awakens cortex via modulation of cross-regional, laminar-specific activity during general anesthesia. Neuron. 2020;106:1–3. [DOI] [PubMed] [Google Scholar]
- 73. Cain JA, Spivak NM, Coetzee JP, et al. . Ultrasonic thalamic stimulation in chronic disorders of consciousness. Brain Stimul. 2021;14:301–303. [DOI] [PubMed] [Google Scholar]
- 74. Yoo S-S, Kim H, Min B-K, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport. 2011;22:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boly M, Massimini M, Tsuchiya N, Postle BR, Koch C, Tononi G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J Neurosci. 2017;37:9603–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sitt JD, King J-R, El Karoui I, et al. . Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(Pt 8):2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lehembre R, Marie-Aurélie B, Vanhaudenhuyse A, et al. . Resting-state EEG study of comatose patients: A connectivity and frequency analysis to find differences between vegetative and minimally conscious states. Funct Neurol. 2012;27:41–47. [PMC free article] [PubMed] [Google Scholar]
- 78. Haugg A, Cusack R, Gonzalez-Lara LE, Sorger B, Owen AM, Naci L. Do patients thought to lack consciousness retain the capacity for internal as well as external awareness? Front Neurol. 2018;9:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu X, Zou Q, Hu J, et al. . Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci. 2015;35:12932–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buckner RL, DiNicola LM. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat Rev Neurosci. 2019;20:593–608. [DOI] [PubMed] [Google Scholar]
- 81. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ-F, et al. . Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090; discussion 1097-1099. [DOI] [PubMed] [Google Scholar]
- 83. Threlkeld ZD, Bodien YG, Rosenthal ES, et al. . Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex. 2018;106:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Norton L, Hutchison RM, Young GB, Lee DH, Sharpe MD, Mirsattari SM. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology. 2012;78:175–181. [DOI] [PubMed] [Google Scholar]
- 85. Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. [DOI] [PubMed] [Google Scholar]
- 86. Laplane D, Degos JD. Motor neglect. J Neurol Neurosurg Psychiatry. 1983;46:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Laplane D, Baulac M, Carydakis C. Motor neglect of thalamic origin. Rev Neurol (Paris). 1986;142:375–379. [PubMed] [Google Scholar]
- 88. Graveleau P, Viader F, Cambier J. Subcortical neglect. Ital J Neurol Sci. 1986;7:573–580. [DOI] [PubMed] [Google Scholar]
- 89. Gao Z, Davis C, Thomas AM, et al. . A cortico-cerebellar loop for motor planning. Nature. 2018;563:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chennu S, Annen J, Wannez S, et al. . Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain. 2017;140:2120–2132. [DOI] [PubMed] [Google Scholar]
- 91. Kustermann T, Ata Nguepnjo Nguissi N, Pfeiffer C, et al. . Brain functional connectivity during the first day of coma reflects long-term outcome. NeuroImage Clin. 2020;27:102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O’Donnell A, Pauli R, Banellis L, et al. . The prognostic value of resting-state EEG in acute post-traumatic unresponsive states. Brain Commun. 2021;3:fcab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giacino JT, Sherer M, Christoforou A, et al. . Behavioral recovery and early decision making in patients with prolonged disturbance in consciousness after traumatic brain injury. J Neurotrauma. 2020;37:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state “plus”: Diagnostic criteria and relation to functional recovery. J Neurol. 2020;267:1245–1254. [DOI] [PubMed] [Google Scholar]
- 95. Naccache L. Minimally conscious state or cortically mediated state? Brain. 2018;141:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kondziella D, Menon DK, Helbok R, et al. . A precision medicine framework for classifying patients with disorders of consciousness: Advanced classification of consciousness endotypes (ACCESS). Neurocrit Care. 2021;35(Suppl 1):27–36. [DOI] [PubMed] [Google Scholar]
- 97. Edlow BL, Sanz LRD, Polizzotto L, et al. . Therapies to restore consciousness in patients with severe brain injuries: A gap analysis and future directions. Neurocrit Care. 2021;35(Suppl 1):68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Claassen J, Akbari Y, Alexander S, et al. . Proceedings of the first curing coma campaign NIH symposium: Challenging the future of research for coma and disorders of consciousness. Neurocrit Care. 2021;35(Suppl 1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hammond FM, Katta-Charles S, Russell MB, et al. . Research needs for prognostic modeling and trajectory analysis in patients with disorders of consciousness. Neurocrit Care. 2021;35(Suppl 1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at New York Presbyterian Hospital—Columbia University. Derived data supporting the findings of this study are available from the corresponding author on request.