Abstract
Objective:
Chronic thromboembolic pulmonary hypertension (CTEPH) following pulmonary embolism (PE) is a morbid complication with suboptimal treatment. We aimed to evaluate the biomarker profile and functional outcomes in patients with submassive PE (sPE) treated with catheter directed thrombolysis (CDT) compared to anticoagulation alone (AC).
Design:
This is a secondary biomarker and survey analysis of the SUNSET sPE randomized trial comparing standard CDT to ultrasound assisted thrombolysis in patients with sPE.
Methods:
As part of the SUNSET sPE study, patients who did not receive an intervention were enrolled into the medical (AC) arm. Biomarkers associated with CTEPH in the literature (CCL2, CXCL10, PTX3, GDF-15, RAGE, BCA-1, TFPI) were collected and measured using multiplex assay at diagnosis, discharge, and three-month follow-up. Patients underwent a 6-minute walk test and answered quality of life (QOL) questionnaires (PEmb, UCSDSOBQ, SF36) three months after diagnosis. Comparisons were made using student’s t-tests. Nonparametric tests were used when the distributions were not normal. Significance was set at P≤.05
Results:
72 patients (56±15 years; 40.3% female) were included in the analysis. 53 of these patients underwent CDT, and 19 additional patients were included in the AC arm. Baseline right ventricle-to-left ventricle (RV/LV) ratios were similar between groups (1.8 CDT, 1.7 AC). Survival and complication rates were similar between groups. At discharge, CXCL10 (768.9±148.6 pg/ml vs. 3,032.0±1,201.0 pg/ml; p=.018) and PTX3 (3,203.5±1,298.0 pg/ml vs. 12,716.2±6,961.5 pg/ml; p=.029) were lower in the CDT group, and displayed a quicker return to baseline than AC. This trend, while not significant, was seen in other biomarkers. At three months, 6-minute walking distance and QOL scores were similar between groups.
Conclusion:
In patients with sPE, biomarkers of CTEPH were lower with CDT compared to AC. At three months, both groups demonstrated similar biomarker levels, 6-minute walking distance, and QOL.
Keywords: Pulmonary embolism, chronic thromboembolic pulmonary hypertension, biomarkers, catheter directed thrombolysis
Table of Contents Summary
This secondary analysis of the SUNSET sPE randomized trial compares biomarkers, functional outcomes, and quality of life after treatment of sPE with catheter directed interventions (CDT) or anticoagulation alone. Specifically, biomarkers CXCL10 and PTX3 may be important biomarkers in assessing response to treatment in the setting of sPE.
Introduction
Pulmonary embolism (PE) is an extremely morbid disease process, causing up to 300,000 deaths in the United States.1 A consequence of PE is chronic thromboembolic disease (CTED), which occurs in up to 30% of patients diagnosed with PE2 and includes patients with unresolved embolism, chronic functional limitations, and a decreased quality of life in the absence of pulmonary hypertension at rest.3,4 Etiology and progression to CTED is largely unknown but risks include initial thrombus burden, delay in anticoagulation, impaired fibrinolysis, and impaired arterial remodeling, among other factors5. The most limiting outcome of PE is chronic thromboembolic pulmonary hypertension (CTEPH), which results from incomplete embolic resolution, causing an increase in right ventricular afterload and compromise RV function.6 If left untreated, progressive RV failure and death may ensue.
It is not entirely clear which patients will develop CTED or CTEPH. Only 0.5–4% of patients with acute PE will progress to CTEPH, and time to progression is largely unknown, ranging from 1–15 years after initial PE diagnosis2. However, studies have shown that prior PE is present in 90% of patients with CTEPH,6 with the remaining 10% of patients likely displaying prior subclinical PE. Risk factors for development of CTEPH include larger initial thrombus, time interval between symptom onset and initiation of anticoagulation, hypercoagulable syndrome, and recurrent thrombosis7. Furthermore, PE and CTED affect quality of life,8,9 however predictors of poor quality of life after PE are not available. Given the pathophysiology and consequences of PE are not well understood, we aimed to evaluate the biochemical, physiologic, and functional outcomes of patients treated for PE, in order to better characterize this patient population. Further, we aimed to identify potential biomarkers which may identify patients more likely to progress to CTEPH.
Treatment for acute PE is dictated by severity of disease which is guided by clinical signs, heart strain on ECHO, circulating biomarkers, and hemodynamics.10 Patients with low-risk PE are treated with anticoagulation, while patients with circulatory shock or persistent hypotension are treated with systemic thrombolysis. However, patients with signs of heart strain without hemodynamic compromise are classified as submassive PE (sPE) or intermediate-risk PE. Catheter-directed therapies are often employed in these patients but clear benefits and guidelines are lacking.10–12 The SUNSET sPE trial aimed to evaluate this patient population and compare treatment with standard catheter directed thrombolysis to ultrasound assisted thrombolysis.13 As part of this study, patients who did not receive an intervention were enrolled in an anticoagulation only (AC) arm. The aim of this study was to perform a secondary analysis of the SUNSET sPE trial in order to measure the effects CDT on clinical, biochemical, physiological, and functional outcomes following sPE compared to those with AC alone. We hypothesized that CDT could optimize these parameters in patients with sPE.
Methods
This is a secondary analysis of the SUNSET sPE randomized clinical trial. SUNSET sPE was a multi-center, randomized, head-to-head, single-blind trial (Clinical trial ID: NCT02758574) of standard CDT versus ultrasound assisted thrombolysis for the treatment of submassive pulmonary embolism (sPE). The study was approved by the Institutional Review Boards of all participating sites as previously described.13 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.14
Patient population and study design
Details regarding randomization, treatment allocation, and procedural details are described elsewhere.13 Briefly, all patients were evaluated by both the interventional team and the pulmonology team as part of a multidisciplinary pulmonary embolism response team. Submassive PE was diagnosed by a combination of 1) PE noted on computed tomography angiography (CTA); 2) right ventricular (RV) strain diagnosed with either RV-to-left ventricular diameter (RV/LV) ratio >1 on CTA or transthoracic echocardiography and/or elevated cardiac biomarkers (troponin or brain natriuretic peptide, and 3) absence of circulatory shock as defined by cardiac arrest, persistent hypotension (two consecutive readings of systolic blood pressure <90mmHg), or vasoactive drug requirement. Exclusion criteria were as follows: age <18 years old, symptoms >14 days, elevated bleeding risk (prior intracranial hemorrhage, intracranial cerebrovascular disease or neoplasm, ischemic stroke within three months, aortic dissection, active bleeding, recent spinal or brain surgery, recent closed head or facial trauma, participation in any other drug or device study, life expectancy <90 days, and inability to comply with study assessments. As a secondary analysis of the SUNSET sPE trial, patients who were not considered by their treating physician, or refused to undergo intervention, were enrolled in the medical arm of the study. These patients were treated with anticoagulation monotherapy.
Patients were followed for three months after PE diagnosis. Follow-up imaging was determined by current guidelines15,16. Every patient enrolled in the SUNSET sPE trial was followed by a single pulmonologist (BRL). At time of diagnosis and three-month follow-up, physiologic outcomes including oxygen requirement and RV/LV diameter ratio were measured. RV/LV ratios were measured from initial CTA by a study member blinded to treatment group. Six-minute walk tests were also performed at three-month follow-up.
At time of diagnosis, discharge, and three-month follow-up, biomarkers that have previously been associated with pulmonary hypertension (MCP-1, CXCL10, PTX3, GDF-15, RAGE, BCA-1) were measured from patient plasma using a customized Luminex multiplex assays (R & D System). The biomarkers that were chosen are experimental and being investigated in pulmonary hypertension research17–20. While they are not immediately available to practice providers currently, they represent potential biomarkers for measuring treatment choice and response.
Finally, three months after diagnosis, quality of life was measured using three different questionnaires: the PEmb questionnaire, which measures quality of life in patients diagnosed with PE,8,21 University of California San Diego Shortness of Breath questionnaire (UCSDSOBQ), which assesses self-reported shortness of breath with activities of daily living,22 and SF-36 questionnaire, which is a global measure of health related quality of life.23
Outcomes
The primary outcome for this secondary analysis was biomarker profile at discharge and three-month follow-up. Secondary outcomes included physiologic outcomes including RV/LV ratio resolution and three-month six-minute walk test. We also analyzed quality of life three months after diagnosis as measured by PEmb, SF-36, and UCSDSOBQ scores.
Statistical analysis
Patient demographics, presenting characteristics, biomarker profiles, and physiologic outcomes were represented using descriptive statistics. Categorical variables were presented as frequencies (%) and were compared using chi-square or fisher exact test. Continuous variables were presented as means and standard deviations (SD) or medians and interquartile ranges (IQRs) and were compared using t test or Wilcoxon rank-sum test. PEmb quality of life score and SF-36 scores were displayed as percent maximum score as previously described9 and presented as medians and IQRs. Statistical significance was defined at a 2-sided P value <0.05. Stata SE 16 (College Station, TX) was used for all statistical analyses.
Results
Eighty-one patients were initially enrolled in the SUNSET sPE trial. 72 of these patients were included in the biomarker subgroup, 53 patients underwent CDT, while 19 patients were treated with anticoagulation alone (Figure 1).
Figure 1. Flow chart displaying study enrollment and outcome measurements.
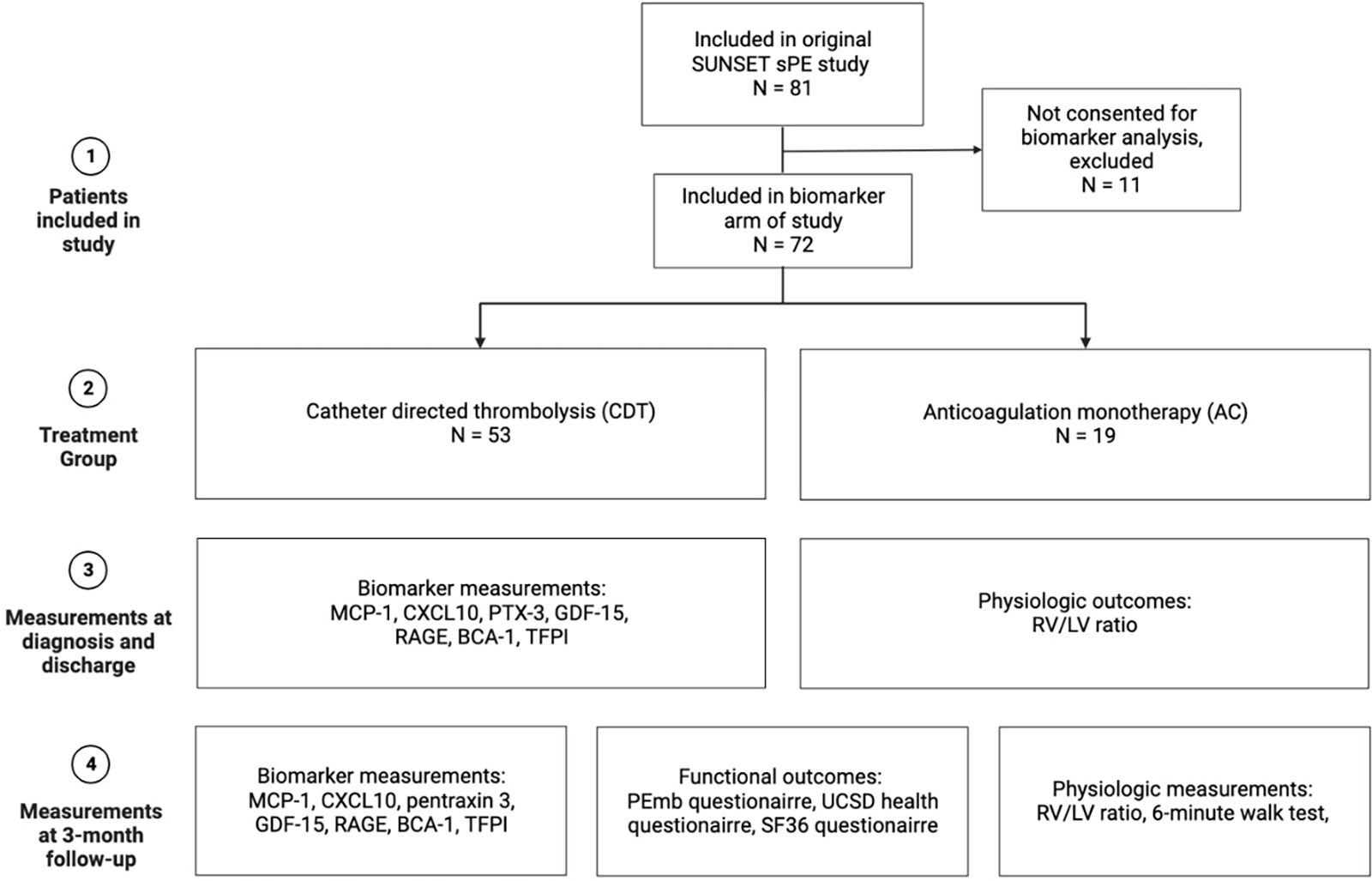
Figure was constructed using biorender.com.
Clinical parameters
Age, gender, and comorbidities were similar between CDT and AC groups. Baseline RV/LV ratio, troponin, d-dimer, and brain natriuretic peptide were also similar between groups (Table 1). Treatment for the CDT group consisted of an average of 13.0±5.8 hours of lysis, 20.0±6.9mg of tissue plasminogen activator (tPA), with the 47 (88.7%) patients receiving a bolus dose. Patients in the AC group were started on a heparin drip (19 patients, 100.0%) and transitioned to apixaban (13 patients, 68.4%), rivaroxaban (3 patients, 15.8%) or warfarin (3 patients, 15.8%). Number of days admitted to the ICU was higher in the CDT group (CDT 2.5±1.4 days vs. AC 1.6±1.4 days; p=0.01), likely due to institutional protocol requiring ICU admission due to the catheter placement. Overall length of hospital stay did not differ between groups (CDT 5.7±2.9 days vs. AC 4.9±3.0 days; p=0.29). There were no complications due to treatment in the AC group. In the CDT group, there were four complications due to treatment, with one major complication which was a hemorrhagic stroke. The patient did not have persistent functional deficits at three-month follow-up. There were no mortalities at three months in either group.
Table 1.
Patient demographics and risk factors.
| Catheter directed thrombolysis N=53 | Anticoagulation Monotherapy N=19 | p value | |
| Age | 55 ± 15 | 61 ± 14 | .08 |
| Male | 32 (60) | 11 (58) | .17 |
| White race | 45 (85) | 18 (95) | .56 |
| Body mass index, kg/m2 | |||
| Coronary artery disease | 9 (17) | 6 (32) | .19 |
| Congestive heart failure | 1 (2) | 1 (5) | .45 |
| Hypertension | 24 (45) | 11 (58) | .38 |
| Pulmonary disease | 4 (8) | 3 (16) | .31 |
| Known hypercoagulable disease | 2 (4) | 1 (5) | .81 |
| Current smoking | 11 (21) | 1 (5) | .11 |
| Recent travel* | 4 (8) | 1 (5) | .72 |
| Recent trauma* | 2 (4) | 0 (0) | .39 |
| Recent surgery* | 13 (25) | 4 (21) | .64 |
| Current malignancy | 2 (4) | 0 (0) | .39 |
| Current oral contraceptive use | 4 (8) | 1 (5) | .72 |
| Glomerular filtration rate | 78.3±27.4 | 77.4±23.1 | .55 |
| Troponin, ug/L† | 0.7 ± 1.6 | 0.6 ± 0.7 | .83 |
| Brain natriuretic peptide, ng/L† | 340.9 ± 315.6 | 257.9 ± 246.9 | .32 |
| D-dimer, † | 15.0 ± 13.4 | 26.6 ± 7.9 | .15 |
| Concomitant DVT | 34 (64) | 13 (68) | .40 |
| Baseline RV/LV ratio on CT | 1.8 ± 0.4 | 1.7 ± 0.3 | .32 |
Values are mean±SD or n (%);
Within 30 days of admission;
Highest value within 24 hours of admission; CT = computed tomography; DVT = deep vein thrombosis; LV = left ventricular; RV = right ventricular. Categorical variables were compared using the Fisher exact test and continuous variables were compared using the Wilcoxon rank-sum test
Biomarker analysis
At time of diagnosis, before treatment with either CDT or AC, there were no significant differences in any circulating biomarker. At time of discharge, there were two biomarkers that displayed different trends from their levels before treatment in CDT compared to AC (Figure 2). CXCL10, an inflammatory chemokine and a biomarker in the development of heart failure and adverse cardiac remodeling24, was elevated higher in the AC group (3,032.0±1,201.0pg/ml) compared to the CDT group which was slightly reduced (768.9±148.6pg/ml) at discharge (p=0.02). Additionally, circulating PTX3, an acute phase reaction protein associated with the prediction and progression of pulmonary hypertension25,26, was dramatically reduced in the CDT group than the AC group at discharge (CDT 3,203.5±1,298.0pg/ml vs. AC 12,716.2±6,961.5pg/ml; p=0.03). From time of diagnosis to time of discharge, CXCL10 was reduced in the CDT group by 37% whereas it had increased in the AC group by 170%. Similarly, but less pronounced, PTX3 was reduced by 74% at time of discharge in CDT whereas it had increased by 3% in the AC group. CXCL10 and PTX3 were reduced to lower than their pretreatment levels in both groups by three-month follow-up (Figure 2A–B). Other markers of chronic inflammation, including RAGE, MCP-1, BCA-1, and GDF-15 peaked at discharge, and decreased over time similarly in both groups (Figure 2C–F).
Figure 2. Circulating concentrations of CXCL10 and PTX 3 reduce earlier following catheter directed thrombolysis (CDT) compared to anticoagulation along (AC).
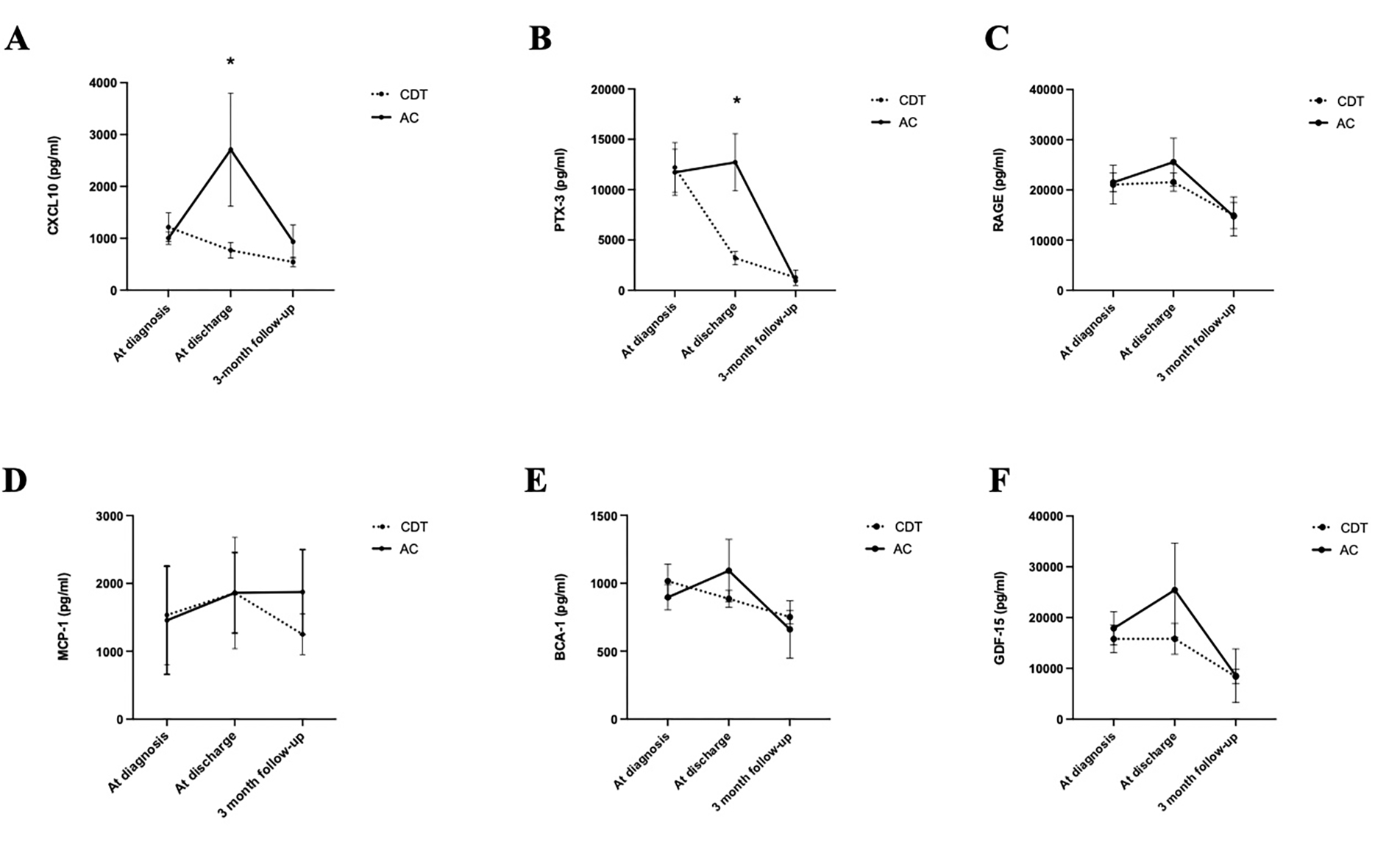
(A) Circulating concentration of CXCL10, (B) PTX-3, (C) RAGE, (D) MCP-1, (E) BCA-1, and (F) GDF-15. All concentrations result in pg/ml. *p<.05, error bars represent standard error of the mean (SEM).
Physiologic outcomes
At three months, there was no difference in self-reported dyspnea at rest between AC (10.5%) and CDT (11.3%; p=0.91). While the CDT group did trend towards a higher reduction in RV/LV ratio at three months, this difference was not statistically significant. The reduction of RV/LV ratio in the AC group was 0.4±0.4 and in the CDT group, the average reduction in RV/LV ratio was 0.6±0.4 (p=0.39). Both groups demonstrated similar 6-minute walking distance at three-month follow-up (Average distance: AC 306.8±46.8 meters vs. CDT 320.0±76.6 meters; p=0.70). Physiologic outcomes are displayed in Figure 3A–B.
Figure 3. Short term physiologic outcomes including RV/LV ratio resolution and six-minute do not change between groups.
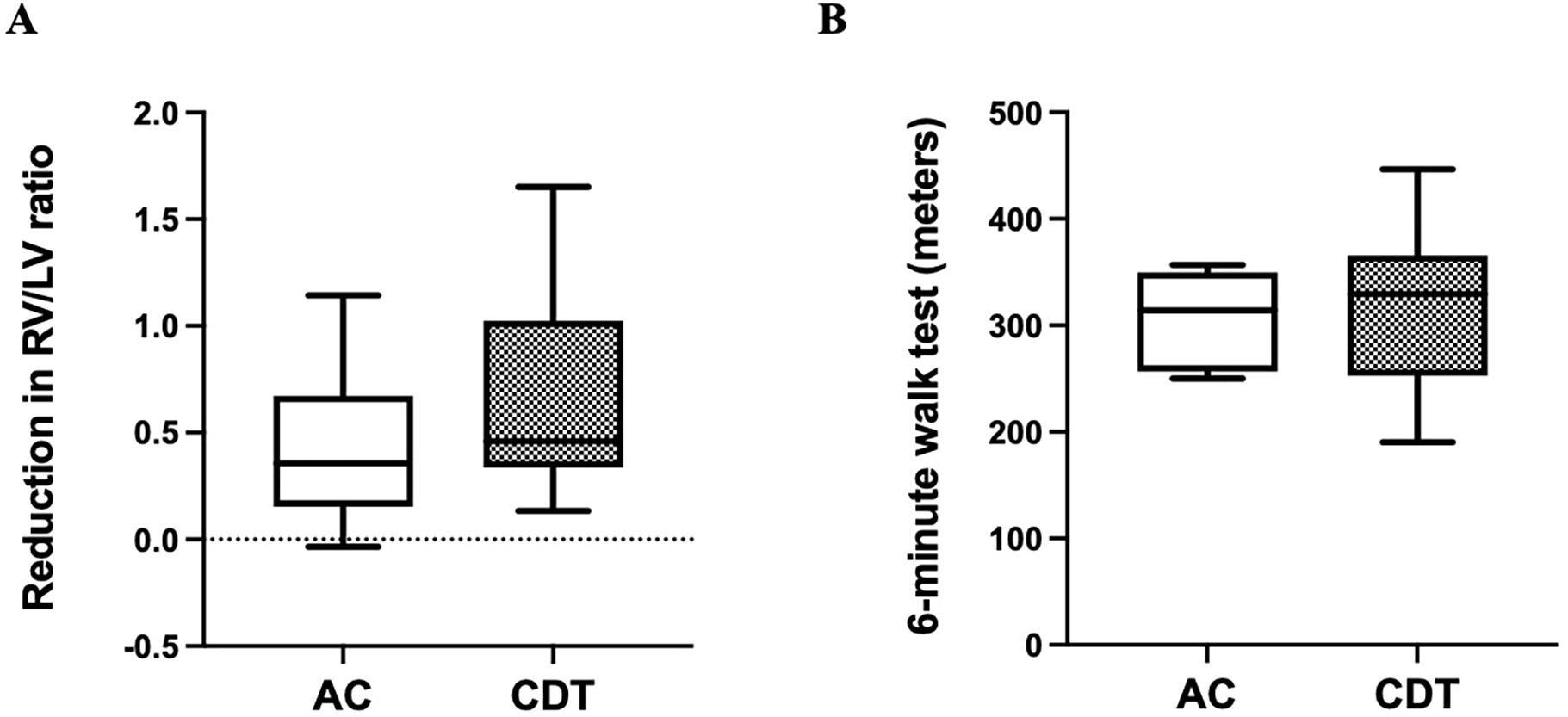
(A) Reduction in right ventricle (RV) to left ventricle (LV) diameter ratio at time of diagnosis compared to the measurement at three-month follow-up. (B) Six-minute walk test distance completed at three-month follow-up. Error bars represent standard error of the mean (SEM).
Quality-of-life
With regards to quality-of-life metrics, there were also no significant differences in functional outcomes calculated by standardized quality of life scores. UCSD shortness of breath questionnaire demonstrated similar scores between groups (AC 6.5, 3.3–17.3 vs. CDT 7.0, 2.8–18.3; p=0.73). Similarly, each domain of the SF-36 score were similar between groups (general health: AC 65.0, 45.0–75.0 vs. CDT 65.0, 40.0–77.5; p=0.86). The PEmb quality of life questionnaire also showed no significant differences across all domains (total score: AC 27.1, 11.4–37.2 vs. CDT 25.8, 3.8–40.5; p=0.45). (Figure 4A–C).
Figure 4. Short term quality of life does not differ between catheter directed thrombolysis (CDT) and anticoagulation monotherapy (AC).
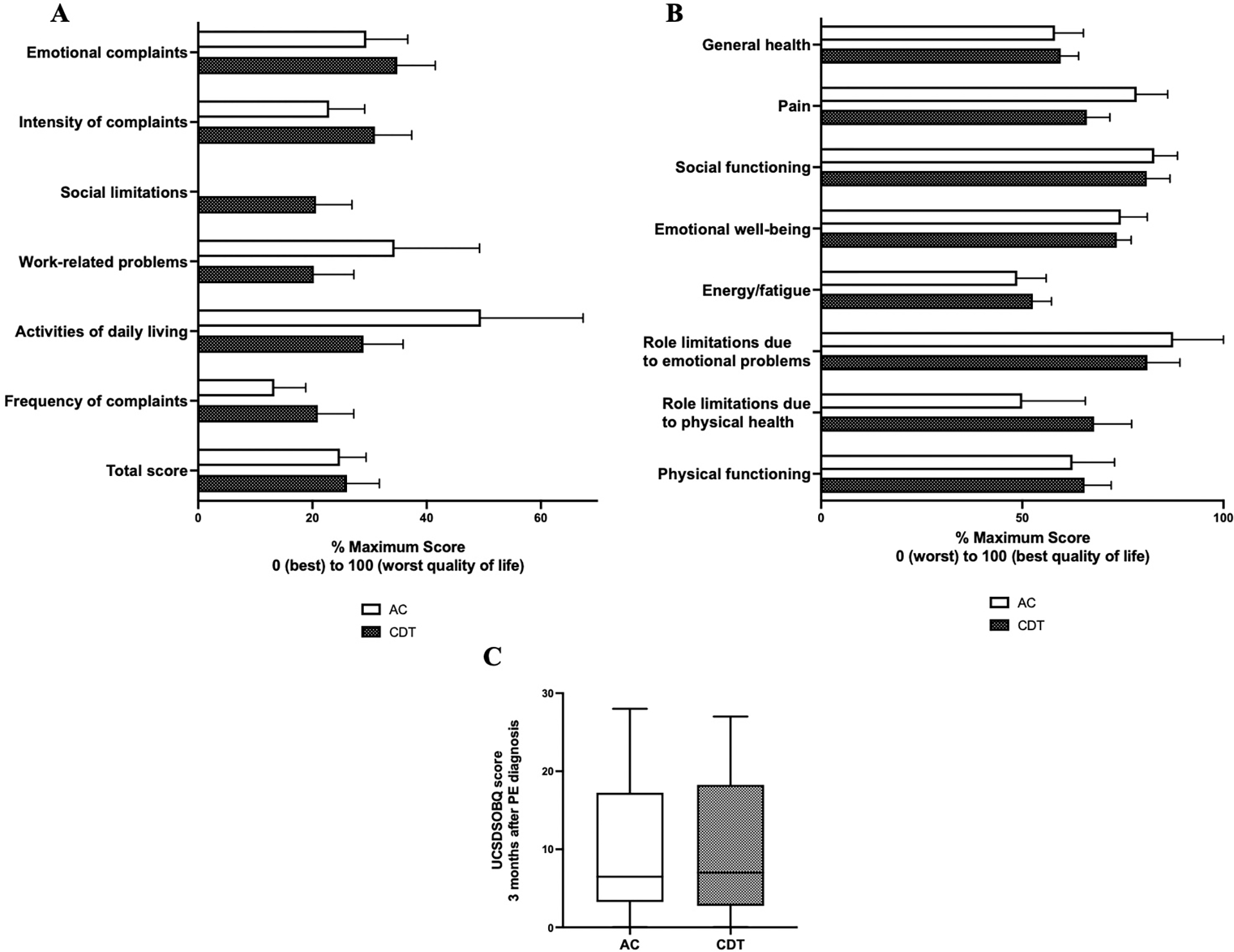
(A) Transformed PEmb quality of life scores three months after pulmonary embolism diagnosis. Higher scores represent worse quality of life. (B) SF36 health related quality of life questionnaire score. Higher scores represent better quality of life. (C) University of California San Diego Shortness of Breath questionnaire (UCSDSOBQ) score three months after pulmonary embolism diagnosis. Higher scores represent more debilitating shortness of breath. Error bars represent standard error of the mean (SEM).
Discussion
In this secondary analysis of the SUNSET sPE randomized control trial, biomarker trends in patients presenting with submassive PE differed between patients who underwent catheter directed therapy compared to anticoagulation alone. Specifically, CXCL10 and PTX3 levels were reduced at an earlier time point in patients who underwent CDT compared to patients in the AC arm.
Clinical outcome measurements between these two groups were similar in short term follow-up. Residual oxygen requirement and mortality were not significantly different between groups. In our population, while not statistically significant, CDT patients did have lower RV/LV ratios at three-month follow-up. This is consistent with recently reported CANARY trial, where Sadeghipour et al found a lower proportion of patients with a RV/LV ratio of >0.9 at three month follow-up after CDT compared to anticoagulation monotherapy, although not statistically significant27. Ongoing trials such as the HI-PEITHO trial are needed to elucidate short term outcomes in head-to-head analyses of catheter directed therapies and anticoagulation monotherapy.28
CXCL10 is a circulating inflammatory marker that is associated with adverse cardiac remodeling and increased in heart failure24. This has been implicated in both animal models and humans with heart failure29,30. Further, it has been identified as a predictor of CTEPH and a marker of CTEPH severity17. As an inflammatory chemokine, it modulates fibroblast activity and vascular cell function24. As such, its more expeditious decline as seen in the current study suggests there may be an alteration in cardiac remodeling after PE when direct catheter-based therapies are employed. Given increased right atrial pressures and heart strain is a known risk factor for development of CTEPH, a more expeditious return to baseline may augment development of CTEPH. Similarly, PTX3 is an acute phase reaction protein that is a marker for vascular inflammation26. It is associated with the development of heart failure26,31. Further, it also is associated with the development of heart failure after cardiac surgery; with implications that it could be used to predict poor outcomes after cardiac interventions25. In the present study, its prolonged elevation after anticoagulation monotherapy suggests a longer period of vascular inflammation, which may have clinical consequences on long-term development of heart failure. This trend of quicker return to baseline physiology after sPE has been reported previously. In the ULTIMA trial, there was a quicker return to baseline RV/LV ratio with USAT compared to anticoagulation monotherapy32. The implications of this are yet to be fully realized in trials, but a faster reduction in right heart dilation and biomarkers associated with pulmonary hypertension and development of CTEPH suggests a faster reduction in these markers may reduce a patients’ risk of development of CTEPH.
Future studies are needed to evaluate the long-term implications of these changes in circulating biomarker concentrations. If reduction in cardiac-specific biomarkers results in a reduction in CTEPH, these markers should be used for risk stratification, and to evaluate response to therapy. In this study, we did not identify changes to quality of life at three months with CDT versus anticoagulation monotherapy, but longer-term follow-up may identify specific ways in which these alternative therapies change quality of life for patients with submassive PE.
This study has several limitations. The SUNSET sPE trial was initially intended to compare USAT to standard CDT. As such, the study population is not powered to detect significant differences in biomarker profile of CDT compared to AC, and the AC arm included a small fraction of total patients in the study. However, this study does highlight observational trends in a largely hypothesis driving study. Additionally, we are limited by length of follow-up. The current study reports three-month outcomes, whereas much of the chronic sequela of PE may occur beyond that time-point. Additional studies are needed to evaluate long term outcomes following treatment for sPE. Further, this study captures thrombolysis, whereas clinical practice preferences are rapidly pivoting to pharmacochemical thrombectomy strategies for treatment of PE. Future studies are needed to evaluate the effect of immediate thrombus debulking with suction devices on the levels of circulating CXCL10 and PTX3 and cardiac outcomes.
Conclusions
In conclusion, we report clinical, biochemical, and quality of life outcomes following treatment for sPE with CDT versus anticoagulation monotherapy. While short term clinical outcomes are unaffected, markers of long-term morbidity after submassive PE are reduced earlier in patients who underwent CDT. In particular, CXCL10 and PTX3, two biomarkers that have been associated with pulmonary hypertension development and cardiac remodeling, represent potential biomarkers that may be important in assessing response to treatment and long-term effects of PE.
Article Highlights.
Type of Research:
Secondary analysis of prospective randomized study
Key Findings:
This secondary analysis of the SUNSET sPE randomized trial compares biomarkers, functional outcomes, and quality of life after treatment of sPE with catheter directed interventions (CDT) or anticoagulation alone. This study identifies different biomarker profiles in patients undergoing CDT compared to anticoagulation alone.
Take home Message:
This study demonstrates biomarkers that may be useful in assessing response to treatment after sPE, and post-intervention risk stratification.
Footnotes
Declaration of conflicts of interest: This research was supported in part by grant T32HL98036 form the National Heart, Lung, and Blood Institute (Andraska). The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (Andraska). The authors have no competing interests.
Presented at 2022 American Venous Forum; Orlando, Florida
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet (London, England) 2012;379(9828):1835–46. Doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 2.Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev 2014;28(6):221–6. Doi: 10.1016/j.blre.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Matthews DT, Hemnes AR. Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension. Pulm Circ 2016;6(2):145–54. Doi: 10.1086/686011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papamatheakis DG, Poch DS, Fernandes TM, Kerr KM, Kim NH, Fedullo PF. Chronic Thromboembolic Pulmonary Hypertension: JACC Focus Seminar. J Am Coll Cardiol 2020;76(18):2155–69. Doi: 10.1016/j.jacc.2020.08.074. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes T, Planquette B, Sanchez O, Morris T. From Acute to Chronic Thromboembolic Disease. Ann Am Thorac Soc 2016;13 Suppl 3:S207–14. Doi: 10.1513/AnnalsATS.201509-619AS. [DOI] [PubMed] [Google Scholar]
- 6.Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350(22):2257–64. [DOI] [PubMed] [Google Scholar]
- 7.Bonderman D, Wilkens H, Wakounig S, Schäfers H-J, Jansa P, Lindner J, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009;33(2):325–31. Doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 8.Klok FA, Cohn DM, Middeldorp S, Scharloo M, Büller HR, van Kralingen KW, et al. Quality of life after pulmonary embolism: validation of the PEmb-QoL Questionnaire. J Thromb Haemost 2010;8(3):523–32. Doi: 10.1111/j.1538-7836.2009.03726.x. [DOI] [PubMed] [Google Scholar]
- 9.Valerio L, Barco S, Jankowski M, Rosenkranz S, Lankeit M, Held M, et al. Quality of Life 3 and 12 Months Following Acute Pulmonary Embolism: Analysis From a Prospective Multicenter Cohort Study. Chest 2021;159(6):2428–38. Doi: 10.1016/j.chest.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 10.Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing G-J, et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021;160(6):2247–59. Doi: 10.1016/j.chest.2021.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Hennemeyer C, Khan A, McGregor H, Moffett C, Woodhead G. Outcomes of Catheter-Directed Therapy Plus Anticoagulation Versus Anticoagulation Alone for Submassive and Massive Pulmonary Embolism. Am J Med 2019;132(2):240–6. Doi: 10.1016/j.amjmed.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Putnam A, Carey K, Marginean A, Serritella A, Friant J, Blair J, et al. Safety and efficacy of catheter-directed therapy versus anticoagulation alone in a higher-risk acute pulmonary embolism population. J Thromb Thrombolysis 2021;52(4):1151–9. Doi: 10.1007/s11239-021-02481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avgerinos ED, Jaber W, Lacomis J, Markel K, McDaniel M, Rivera-Lebron BN, et al. Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial. JACC Cardiovasc Interv 2021;14(12):1364–73. Doi: 10.1016/j.jcin.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. Doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022:2200879. Doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 16.Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, et al. ERS Statement on Chronic Thromboembolic Pulmonary Hypertension. Eur Respir J 2020:2002828. Doi: 10.1183/13993003.02828-2020. [DOI] [PubMed] [Google Scholar]
- 17.Hong C, Lu J, Chen R, Liu H, Chen H, Wu X, et al. CXCL10 levels in diagnosis and improved hemodynamics in patients with chronic thromboembolic pulmonary hypertension undergoing balloon pulmonary angioplasty. Pulm Circ 2022;12(2):e12091. Doi: 10.1002/pul2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch K, Nolley S, Ralph DD, Zheng Y, Altemeier WA, Rhodes CJ, et al. Circulating markers of inflammation and angiogenesis and clinical outcomes across subtypes of pulmonary arterial hypertension. J Hear Lung Transplant Off Publ Int Soc Hear Transplant 2023;42(2):173–82. Doi: 10.1016/j.healun.2022.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito A, Tanabe N, Jujo T, Shigeta A, Sugiura T, Sakao S, et al. Pentraxin3 in chronic thromboembolic pulmonary hypertension: a new biomarker for screening from remitted pulmonary thromboembolism. PLoS One 2014;9(11):e113086. Doi: 10.1371/journal.pone.0113086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, et al. Human pentraxin 3 (PTX3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension. PLoS One 2012;7(9):e45834. Doi: 10.1371/journal.pone.0045834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Es J, den Exter PL, Kaptein AA, Andela CD, Erkens PMG, Klok FA, et al. Quality of life after pulmonary embolism as assessed with SF-36 and PEmb-QoL. Thromb Res 2013;132(5):500–5. Doi: 10.1016/j.thromres.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113(3):619–24. Doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 23.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83. [PubMed] [Google Scholar]
- 24.Chen B, Frangogiannis NG. Chemokines in Myocardial Infarction. J Cardiovasc Transl Res 2021;14(1):35–52. Doi: 10.1007/s12265-020-10006-7. [DOI] [PubMed] [Google Scholar]
- 25.Song Y-K, Yuan H-X, Jian Y-P, Chen Y-T, Liang K-F, Liu X-J, et al. Pentraxin 3 in Circulating Microvesicles: a Potential Biomarker for Acute Heart Failure After Cardiac Surgery with Cardiopulmonary Bypass. J Cardiovasc Transl Res 2022. Doi: 10.1007/s12265-022-10253-w. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, He R, Man C, Gong D. Utility of Elevated Pentraxin-3 Level as Inflammatory Marker for Predicting Adverse Outcomes in Patients With Acute Coronary Syndrome: A Meta-Analysis. Front Cardiovasc Med 2021:736868. Doi: 10.3389/fcvm.2021.736868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeghipour P, Jenab Y, Moosavi J, Hosseini K, Mohebbi B, Hosseinsabet A, et al. Catheter-Directed Thrombolysis vs Anticoagulation in Patients With Acute Intermediate-High-risk Pulmonary Embolism: The CANARY Randomized Clinical Trial. JAMA Cardiol 2022. Doi: 10.1001/jamacardio.2022.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klok FA, Piazza G, Sharp ASP, Ní Ainle F, Jaff MR, Chauhan N, et al. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: Rationale and design of the HI-PEITHO study. Am Heart J 2022;251:43–53. Doi: 10.1016/j.ahj.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Altara R, Mallat Z, Booz GW, Zouein FA. The CXCL10/CXCR3 Axis and Cardiac Inflammation: Implications for Immunotherapy to Treat Infectious and Noninfectious Diseases of the Heart. J Immunol Res 2016;2016:4396368. Doi: 10.1155/2016/4396368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altara R, Gu Y-M, Struijker-Boudier HAJ, Thijs L, Staessen JA, Blankesteijn WM. Left Ventricular Dysfunction and CXCR3 Ligands in Hypertension: From Animal Experiments to a Population-Based Pilot Study. PLoS One 2015;10(10):e0141394. Doi: 10.1371/journal.pone.0141394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath M, Romaine SPR, Koekemoer A, Hamby S, Webb TR, Nelson CP, et al. Whole blood transcriptomic profiling identifies molecular pathways related to cardiovascular mortality in heart failure. Eur J Heart Fail 2022;24(6):1009–19. Doi: 10.1002/ejhf.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129(4):479–86. Doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]


