Abstract
Background
In patients with Parkinson’s Disease (PD), two distinct motor subtypes, tremor dominant (TD) and postural instability and gait difficulty (PIGD), can be differentiated using Unified Parkinson’s Disease Rating Scale (UPDRS) sub-scores. This post hoc analysis of pooled data from eight pivotal studies examined the effect of treatment with istradefylline, a selective adenosine A2A receptor antagonist, on these subtypes.
Methods
In eight randomized, placebo-controlled phase 2b/3 trials, patients on levodopa with carbidopa/benserazide experiencing motor complications received istradefylline (20 or 40 mg/day) or placebo for 12 or 16 weeks. TD subtype was defined by the UPDRS II/III items kinetic and postural tremor in right/left hand and (resting) tremor in the face, lips, chin, hands, or feet; PIGD items were freezing, walking, posture, gait, and postural instability. The ratio of mean scores from TD:PIGD items determined subtype (TD [TD:PIGD ratio ≥ 1.5], PIGD [TD:PIGD ratio ≤ 1.0], mixed-type [ratio 1–1.5]).
Results
In total, 2719 patients were included (PIGD, n = 2165; TD, n = 118; mixed-type, n = 188; not evaluable, n = 248). Among TD subtype patients, the least-squares mean change from baseline versus placebo in UPDRS II/III TD-related total score was significant at 20 mg/day istradefylline (−2.21; 95 % CI, −4.05 to −0.36; p = 0.02). For PIGD subtype patients, there was a significant difference from placebo in UPDRS II/III PIGD-related total score at 40 mg/day istradefylline (−0.25; −0.43 to −0.06; p = 0.01).
Conclusions
The data from this analysis of UPDRS-based motor subtypes suggest that istradefylline can improve motor disability in PD patients with motor fluctuations regardless of PD subtype. Future research should characterize the effects of istradefylline on tremor.
Keywords: Parkinson’s disease, Tremor dominant, Postural instability and gait difficulty, Istradefylline, Motor symptoms
1. Introduction
Istradefylline is a nondopaminergic, selective adenosine A2A receptor antagonist indicated in the US and Japan as adjunctive treatment to levodopa (LD)/decarboxylase inhibitor in adults with Parkinson’s disease (PD) experiencing OFF episodes [1]. In patients with PD, the balance between the indirect and direct gamma-amino butyric acid (GABA)-ergic output pathways changes as a result of degeneration of nigrostriatal neurons and a decline in dopaminergic transmission. These changes result in excessive inhibitory signaling from the indirect pathway to the globus pallidus external segment (GPe), thereby increasing GABAergic inhibition of the thalamus and brainstem and suppressing movement [2]. Additionally, excitatory signaling from A2A receptors—which are highly localized to the caudate nucleus, putamen, and nucleus accumbens—increases, while inhibitory signaling from dopamine D2 receptors decreases, augmenting the imbalance between the direct and indirect pathways [3]. Istradefylline antagonizes adenosine A2A receptors in the striatum, improving motor control by reducing excessive inhibition from the indirect pathway. This mechanism is thought to restore balance between the direct and indirect pathways [3].
Istradefylline has demonstrated efficacy in LD- and carbidopa/benserazide-treated patients with PD experiencing motor fluctuations. In a large pooled analysis of eight placebo-controlled randomized clinical trials, once-daily istradefylline (20 or 40 mg/day) significantly reduced OFF time and increased ON time without troublesome dyskinesia relative to placebo. Additionally, istradefylline treatment was safe and well tolerated; the most common adverse event was dyskinesia for both dose levels [4]. PD can be categorized into two distinct motor subtypes based on Unified Parkinson’s Disease Rating Scale (UPDRS) sub-scores: tremor dominant (TD) and postural instability and gait difficulty (PIGD); the distinction between these subtypes is important, as it can inform on clinical progression and effective treatment strategies [5]. TD is typically associated with kinetic and postural tremors in the hands and tremors while at rest in the face, lips, chin, arms, and/or legs. In contrast, PIGD characteristics include freezing of gait (FOG), difficulty walking, and/or postural instability [5], [6]. Importantly, patients with the PIGD subtype may have more rapid disease progression, experience greater functional impairment, and be less responsive to LD therapy than TD subtype patients [5], [6]. This post hoc pooled analysis was performed to investigate the efficacy of istradefylline as an adjunct to LD as measured by changes in the UPDRS Part II and III total scores related to the TD and PIGD motor subtypes.
2. Methods
2.1. Patients and studies
The methodology and study details of the studies included in this pooled analysis have been previously reported [4]. Briefly, eight randomized, placebo-controlled phase 2b/3 studies (NCT00199394, NCT00199407, NCT00199420, NCT00455507, NCT00456586, NCT00456794, NCT00955526, NCT01968031) were conducted in North America, Japan, or internationally. These studies enrolled patients with PD experiencing motor fluctuations (end-of-dose wearing-off) during treatment with LD/decarboxylase inhibitors, with or without other anti-PD medications. Patients were randomized to receive istradefylline or placebo; while the studies included several doses of istradefylline (10, 20, 40, or 60 mg/day), only the 20 and 40 mg treatment arms were selected for inclusion in the pooled analysis, as the 20 and 40 mg doses are approved for use in clinical practice and were used by the majority of the patients in the eight randomized studies [7].
Data from all eight randomized controlled trials were pooled for this post hoc analysis. The studies were specifically designed to share common methodology, allowing data pooling [4]. The TD and PIGD disease subtype groups were established post hoc using the ratio of mean baseline TD:PIGD UPDRS scores. TD items were defined as kinetic or postural tremor in both the right and left hand (UPDRS Part III-ON items 21A and B) and tremor while at rest in the face, lips, chin, hands, or feet (UPDRS Part III-ON items 20A-20E) [5], [8]. PIGD items were defined as freezing (UPDRS II-ON item 14), walking (UPDRS II-ON item 15), posture (UPDRS III-ON item 28), gait (UPDRS III-ON, item 29), and postural stability (UPDRS III-ON, item 30) [5], [8]. Ratio of mean baseline TD:PIGD scores defined the motor subtype as TD (TD:PIGD ratio ≥ 1.5), PIGD (TD:PIGD ratio ≤ 1.0), or mixed (TD:PIGD ratio > 1–<1.5) [5], [8].
Because these were not analyses of pre-specified endpoints, no statistical analysis plan was established. A two-way analysis of covariance (ANCOVA) model with baseline assessment as a covariate and treatment group and study as fixed-effect terms was used to compare least-squares (LS) mean change from baseline in the total scores for TD- and PIGD-related UPDRS II/III items between motor subtype groups. P-values were based on paired comparisons between each istradefylline treatment arm and placebo. Mean values are presented as mean (SD) unless otherwise indicated.
3. Results
3.1. Patients
Of 2719 patients included in this analysis of PD patients experiencing wearing-off, 118 (4.3 %) were TD type, 2165 (79.6 %) were PIGD type, and 188 (6.9 %) were mixed; 248 (9.1 %) patients were not evaluable on the basis of TD and/or PIGD scores. Baseline characteristics between subgroups are shown in Table 1. Mean age was 61.4 years (SD, 9.11) in the TD subgroup and 64.7 (SD, 8.72) in the PIGD subgroup; the majority of patients were male, and patients had a mean baseline total OFF time of 6.61 h/day (SD, 2.44) in the TD subgroup and 6.14 (SD, 2.41) in the PIGD subgroup. At baseline, the mean (SD) time since initiation of LD was 8.22 years (4.56; n = 1231) in patients with the PIGD subtype versus 6.81 years (4.98; n = 85) in those with the TD subtype. Additionally, mean (SD) time since onset of motor complications was 4.33 years (3.79; n = 2159) among patients with PIGD-type disease compared with 2.93 years (2.37; n = 117) in patients with the TD subtype.
Table 1.
Baseline characteristics, demographics, and concomitant medications.
|
TD Subtype |
PIGD Subtype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 46) | Istradefylline |
Total (n = 118) | Placebo (n = 792) | Istradefylline |
Total (n = 2165) | |||
| 20 mg/day (n = 27) |
40 mg/day (n = 45) |
20 mg/day (n = 679) |
40 mg/day (n = 694) |
|||||
| Age, years Mean (SD) [Range] |
62.8 (8.96) [42–80] |
62.5 (9.96)[43–80] | 59.4 (8.54) [35–79] |
61.4 (9.11) [35–80] |
64.5 (8.77) [36–87] |
65.0 (8.97) [35–87] |
64.6 (8.40) [38–85] |
64.7 (8.72) [35–87] |
| Sex Male, n (%) |
33 (71.7) | 15 (55.6) | 32 (71.1) | 80 (67.8) | 448 (56.6) | 385 (56.7) | 388 (55.9) | 1221 (56.4) |
| BMI, kg/m2 Mean (SD) [Range] |
25.63 (4.714) [17.9–43.3] |
27.08 (6.707) [21.3–54.4] |
26.48 (4.544) [17.4–36.5] |
26.29 (5.165) [17.4–54.4] |
25.51 (5.008) [15.3–46.9] |
25.62 (5.132) [15.3–49.3] |
25.43 (5.341) [14.2–57.2] |
25.52 (5.154) [14.2–57.2] |
| Total OFF time, hours/daya Mean (SD) [Range] |
7.03 (1.81) [3.8–11.8] |
6.26 (3.09) [2.2–14.8] |
6.39 (2.55) [2.5–14.5] |
6.61 (2.44) [2.2–14.8] |
6.20 (2.35) [0.8–14.8] |
6.20 (2.53) [1.0–17.8] |
6.04 (2.35) [0–16.3] |
6.14 (2.41) [0–17.8] |
| Total daily dose of LD at study entry, mg Mean (SD) [Range] |
n = 46 | n = 27 | n = 45 | n = 118 | n = 792 | n = 677 | n = 693 | n = 2162 |
| 724.46 (416.85) [125–1750] |
531.94 (272.73) [150–1263] |
615.56 (335.42) [150–1600] |
638.88 (362.49) [125–1750] |
688.81 (383.79) [25–3200] |
677.95 (355.19) [150–2500] |
645.57 (372.29) [105–4800] |
671.55 (371.62) [25–4800] |
|
| Time since PD diagnosis, years Mean (SD)b |
n = 40 | n = 23 | n = 38 | n = 101 | n = 616 | n = 511 | n = 510 | n = 1637 |
| 7.51 (5.34) | 8.93 (6.43) | 7.69 (3.87) | 7.90 (5.11) | 9.03 (4.67) | 9.16 (4.93) | 8.60 (4.51) | 8.94 (4.71) | |
| Time since initiation of LD, years Mean (SD)b |
n = 36 | n = 19 | n = 30 | n = 85 | n = 476 | n = 355 | n = 400 | n = 1231 |
| 7.30 (5.56) | 6.28 (5.00) | 6.57 (4.29) | 6.81 (4.98) | 8.26 (4.41) | 8.37 (4.69) | 8.03 (4.62) | 8.22 (4.56) | |
| Time since onset of motor complications, Mean (SD) |
n = 46 | n = 27 | n = 44 | n = 117 | n = 790 | n = 677 | n = 692 | n = 2159 |
| 2.73 (2.54) | 2.66 (2.17) | 3.29 (2.31) | 2.93 (2.37) | 4.35 (3.75) | 4.38 (3.79) | 4.24 (3.81) | 4.33 (3.79) | |
| Concomitant medications, n (%) DAs COMT inhibitors MAO-B inhibitors |
24 (52.2) 14 (30.4) 8 (17.4) |
19 (70.4) 7 (25.9) 5 (18.5) |
24 (53.3) 15 (33.3) 10 (22.2) |
67 (56.8) 36 (30.5) 23 (19.5) |
614 (77.5) 238 (30.1) 233 (29.4) |
546 (80.4) 292 (43.0) 203 (29.9) |
527 (75.9) 225 (32.4) 217 (31.3) |
1687 (77.9) 755 (34.9) 653 (30.2) |
Not included: Patients with mixed subtype (n = 188) and patients not evaluable on the basis of TD and/or PIGD scores (n = 248).
COMT, catechol-O-methyl transferase; DA, dopamine agonist; LD, levodopa; MAO-B, monoamine oxidase type B; PIGD, postural instability and gait difficulty; SD, standard deviation; TD, tremor dominant.
For studies in which the primary endpoint (OFF time) was recorded as the percentage of awake time (eg, % OFF), data were converted to hours/day for pooling.
Data not collected in all eight studies.
3.2. UPDRS scores
At baseline, combined mean UPDRS Parts II and III total scores were comparable between TD and PIGD patients treated with istradefylline at 20 mg/day (30.41 [SD, 12.92] and 30.23 [SD, 14.17], respectively) and 40 mg/day (36.51 [SD, 15.14] and 31.83 [SD, 14.17], respectively; Supplementary Table S1).
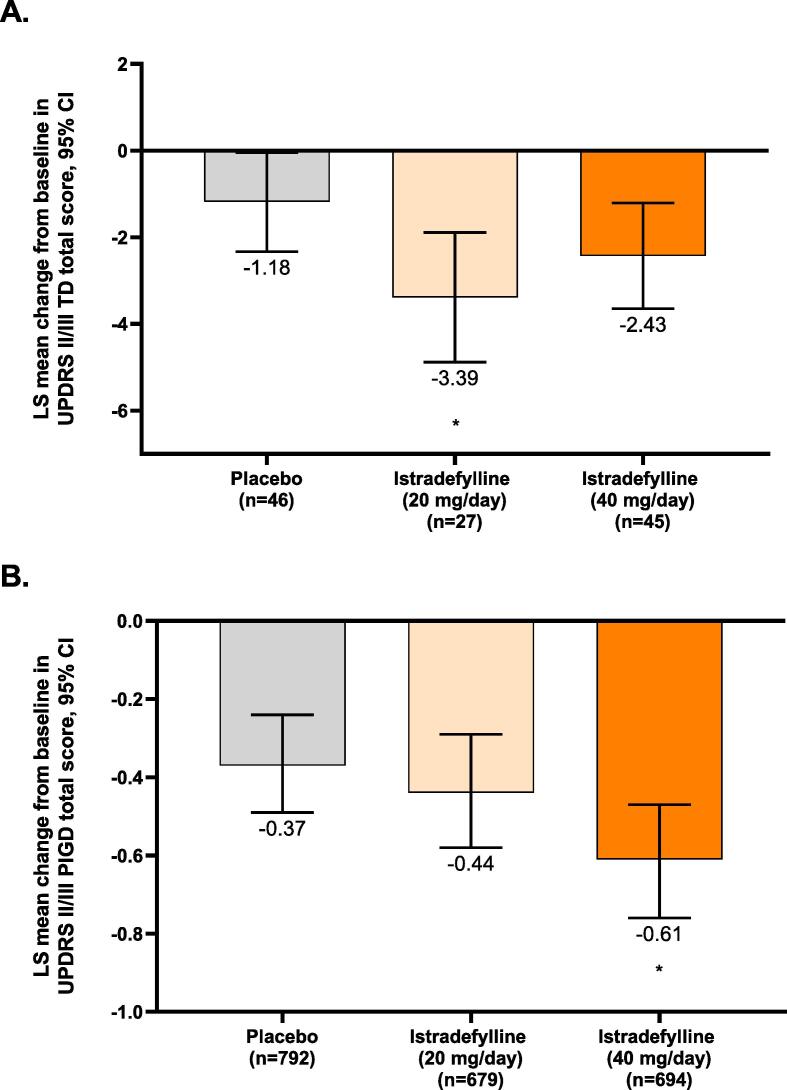
Among patients with the TD subtype, the LS mean changes from baseline in UPDRS II/III TD-related total scores were −1.18 (95 % CI, −2.33 to −0.04) for placebo, −3.39 (−4.88 to −1.89) for istradefylline 20 mg/day, and −2.43 (−3.65 to −1.21) for istradefylline 40 mg/day (Fig. 1A). Patients with the TD subtype exhibited a significant difference in LS mean change from baseline in TD-related UPDRS II/III scores when comparing placebo-treated patients to patients receiving istradefylline 20 mg/day (−2.21; 95 % CI, −4.05 to −0.36; p = 0.02); a similar but non-significant change was observed between patients treated with placebo and istradefylline 40 mg/day (−1.25; −2.87 to 0.37; p = 0.13). Among patients with the PIGD subtype, the LS mean changes from baseline in UPDRS II/III PIGD-related total scores were −0.37 (−0.49 to −0.24) for placebo, −0.44 (−0.58 to −0.29) for istradefylline 20 mg/day, and −0.61 (−0.76 to −0.47) for istradefylline 40 mg/day (Fig. 1B). For patients with the PIGD subtype, the difference in LS mean change from baseline in UPDRS II/III PIGD-related score compared to placebo was not significant for istradefylline 20 mg/day (−0.07; 95 % CI, −0.26 to 0.12; p = 0.46) but reached significance at 40 mg/day (−0.25; −0.43 to −0.06; p = 0.01).
Fig. 1.
LS mean change from baseline in UPDRS II/III total score in patients with (A) TD or (B) PIGD subtypes. *p < 0.05 vs. placebo; **p = 0.01 vs. placebo. P-values were based on paired comparisons between each istradefylline treatment arm and placebo. A two-way ANCOVA model was used with baseline assessment as a covariate and treatment group and study as fixed-effect terms. ANCOVA, analysis of covariance; CI, confidence interval; LS, least-squares; PIGD, postural instability and gait difficulty; TD, tremor dominant; UPDRS, Unified Parkinson’s Disease Rating Scale.
4. Discussion
In this analysis of eight phase 2b/3 studies, treatment with istradefylline at 20 mg/day and 40 mg/day improved UPDRS scores related to the PIGD and TD subtypes, respectively, for patients. This result may be attributable to the A2A receptor location as well as the contribution of adenosine signaling in the entire basal ganglia and its outputs as related to these subtypes. While TD is associated with the subthalamic nucleus (STN) [9], FOG, a symptom associated with PIGD, may occur via the pedunculopontine nucleus (PPN) [10]. The STN is directly affected by the striatopallidal indirect pathway, as it is directly downstream, whereas the PPN is a convergence affected by both the indirect and direct pathways via the output of the basal ganglia (ie, GPi/SNr) [11]. Therefore, given the underlying differences contributing to the two pathways, the PIGD and TD subtypes may respond differently to istradefylline. This possibility is supported by the observations from this post hoc analysis, in which patients with the TD subtype disease appeared more responsive at the lower istradefylline dose and patients with PIGD subtype appeared more responsive at the higher dose with respect to the UPDRS II/III sub-scores related to each of these subtypes. This finding suggests that patients with TD may benefit from istradefylline treatment at 20 mg/day while patients with PIGD may derive the most benefit at 40 mg/day; however, the observed differences were small, and the clinical significance of this finding requires further study. Moreover, evaluation of the istradefylline dose–response relationship is difficult, and results are inconsistent across trials [4]. Caution is advised when interpreting the utility of istradefylline dosages as they pertain to PD motor subtype. Furthermore, because of the small sample size available for the TD subtype, additional investigation is necessary, including any effect that istradefylline treatment may have on disease progression, given the rapid progression associated with PIGD PD [6].
This post hoc analysis included patients with PD who were experiencing wearing-off phenomena. Generally, wearing-off is experienced by patients with mid- to late-stage PD, and this trend is reflected in our post hoc separation of the study population into TD and PIGD subtypes, where the PIGD subtype dominated. The TD subtype is observed more frequently in patients early in the disease course, and observations in the published literature suggest that in some patients, TD and PIGD may actually be sequential stages of PD, with patients moving from TD to PIGD subtype around mid-stage disease, when they are also more likely to experience wearing-off, along with increased impact on activities of daily living (ADLs) [12]. Accordingly, longer mean times since PD diagnosis and onset of motor complications were observed among patients with the PIGD subtype compared to TD in our study population. Our findings may have implications for the impact of motor subtype on ADLs and quality of life (QoL). However, further research is necessary to understand these impacts in patients with PD.
This analysis has several limitations. Due to the post hoc nature of this study and the relatively small number of patients with the TD subtype, the generalizability of these differences may be limited, and any differences in demographic and disease characteristics between the TD and PIGD subtypes have not been addressed. Furthermore, rates of TD are considerably lower in this analysis than in previous publications on PD motor subtypes, although the variability of PD motor subtypes across different studies has been documented [13]. An evaluation of PD subtype consistency among different studies found that the prevalence of different PD subtypes varied widely depending on the algorithm used, possibly owing to the inclusion of different UPDRS items in different algorithms and the somewhat arbitrary nature of cutoffs to determine subtypes [13]. Additionally, the subtype determination algorithm used for this study did not account for akinetic-rigidity characteristics, which, in some publications, are used to define a separate motor subtype [14]. Therefore, additional studies evaluating the effect of istradefylline in patients with various motor subtypes may help further clarify the potential impact of an adenosine A2A antagonist on these symptoms of PD.
This post hoc analysis of patients exhibiting TD or PIGD subtype characteristics at baseline indicates that istradefylline can improve motor disability in PD patients with motor fluctuations regardless of PD subtype.
Funding
This study was funded by Kyowa Kirin, Inc.
CRediT authorship contribution statement
Yasar Torres-Yaghi: Conceptualization, Investigation, Writing – review & editing. Nobutaka Hattori: Investigation, Writing – review & editing. Olivier Rascol: Investigation, Writing – review & editing. Yu Nakajima: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Shelby M. King: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. Akihisa Mori: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Fernando Pagan: Conceptualization, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yasar Torres-Yaghi served as a consultant/speaker for Abbott, AbbVie, Acadia, Amneal, Kyowa Kirin, Teva, and US WorldMeds. Nobutaka Hattori received research funding from/served as an investigator for AbbVie GK, Asahi Kasei, Astellas, Boston Scientific, Dai-Nippon Sumitomo, Daiichi Sankyo, Eisai, FP Pharmaceutical, Kyowa Hakko Kirin, Medtronic, Mitsubishi Tanabe, MiZ, Nihon Medi-physics, Nihon Pharmaceutical, Nippon Boehringer Ingelheim, OHARA, Ono Pharmaceutical, Otsuka, and Takeda; subcontracted trial cases for Biogen Japan, Hisamitsu, and Meiji Seika; did contract research for Biogen Japan and Mitsubishi Tanabe; served as an advisor/consultant for Dai-Nippon Sumitomo; served on advisory boards for Dai-Nippon Sumitomo, Kyowa Hakko Kirin, Otsuka, Sanofi KK, and Takeda; and received honoraria from AbbVie GK, Alexion, Boston Scientific, Dai-Nippon Sumitomo, Daiichi Sankyo, FP Pharmaceutical, Kyowa Hakko Kirin, Lundbeck Japan, Medtronic, MSD KK, Mylan, Nippon Boehringer Ingelheim, Novartis, Otsuka, Pfizer, and Takeda. Olivier Rascol received research funding from/served as an investigator for Agence Nationale de la Recherche (ANR), CHU de Toulouse, European Commission (FP7, H2020), France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, Michael J. Fox Foundation, Programme Hospitalier de Recherche Clinique, and served as an advisor/consultant for AbbVie, Adamas, Acorda, Addex, AlzProtect, ApoPharma, AstraZeneca, Bial, Biogen, Britannia, Bukwang, Cerevel, CleveXel, IRLAB, Lilly, Lundbeck, Lupin, Merck, Mundipharma, NeurATRIS, Neuroderm, Novartis, ONO Pharma, Orion, Osmotica, Oxford Biomedica, Parexel, Pfizer, Prexton, Quintiles, Sanofi, Servier, Sunovion, Theranexus, Takeda, Teva, UCB, Watermark Research, XenoPort, XO, and Zambon. Yu Nakajima is an employee of Kyowa Kirin Co., Ltd. Shelby M. King is a former employee of Kyowa Kirin, Inc. Akihisa Mori is a former employee of Kyowa Kirin Co., Ltd. Fernando Pagan received educational/research grants from Medtronic, Sun Pharma, and US WorldMeds; served as a consultant/speaker for Acadia, Acorda, Amneal, AbbVie, Kyowa Kirin, Merz, Neurocrine, Sunovion, Supernus and US WorldMeds; and is a co-founder and shareholder of KeifeRx.
Acknowledgments
The authors thank Meghan Sullivan, PhD, and Anthony DiLauro, PhD, of MedVal Scientific Information Services, LLC, for medical writing and editorial assistance, which were funded by Kyowa Kirin, Inc. This study was funded by Kyowa Kirin, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2023.100224.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mori A., LeWitt P., Jenner P. In: The Adenosinergic System: A Non-Dopaminergic Target in Parkinson’s Disease. Morelli M., editor. Springer International Publishing; Basel, Switzerland: 2015. The story of istradefylline—the first approved A2A antagonist for the treatment of Parkinson’s disease; pp. 273–289. [Google Scholar]
- 2.Mori A. Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson's disease. Int. Rev. Neurobiol. 2014;119:87–116. doi: 10.1016/B978-0-12-801022-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 3.Jenner P., Mori A., Aradi S.D., Hauser R.A. Istradefylline – a first generation adenosine A2A antagonist for the treatment of Parkinson's disease. Expert Rev. Neurother. 2021;21(3):317–333. doi: 10.1080/14737175.2021.1880896. [DOI] [PubMed] [Google Scholar]
- 4.Hauser R.A., Hattori N., Fernandez H., Isaacson S.H., Mochizuki H., Rascol O., Stocchi F., Li J., Mori A., Nakajima Y., Ristuccia R., LeWitt P. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J. Parkinsons Dis. 2021;11(4):1663–1675. doi: 10.3233/JPD-212672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezvanian S., Lockhart T., Frames C., Soangra R., Lieberman A. Motor subtypes of Parkinson's disease can be identified by frequency component of postural stability. Sensors (Basel, Switzerland) 2018;18(4):1102. doi: 10.3390/s18041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankovic J., Kapadia A.S. Functional decline in Parkinson disease. Arch. Neurol. 2001;58(10):1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- 7.NOURIANZ™ (istradefylline) tablets, for oral use. US prescribing information. Bedminster, NJ: Kyowa Kirin Inc.; May 2020.
- 8.Jankovic J., McDermott M., Carter J., Gauthier S., Goetz C., Golbe L., Huber S., Koller W., Olanow C., Shoulson I., Stern M., Tanner C., Weiner W. The Parkinson Study Group, Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 9.Mure H., Hirano S., Tang C.C., Isaias I.U., Antonini A., Ma Y., Dhawan V., Eidelberg D. Parkinson's disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage. 2011;54(2):1244–1253. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iijima M., Orimo S., Terashi H., Suzuki M., Hayashi A., Shimura H., Mitoma H., Kitagawa K., Okuma Y. Efficacy of istradefylline for gait disorders with freezing of gait in Parkinson's disease: a single-arm, open-label, prospective, multicenter study. Expert Opin. Pharmacother. 2019;20(11):1405–1411. doi: 10.1080/14656566.2019.1614167. [DOI] [PubMed] [Google Scholar]
- 11.Mori A. How do adenosine A2A receptors regulate motor function? Parkinsonism Relat. Disord. 2020;80(Suppl 1):S13–S20. doi: 10.1016/j.parkreldis.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.W., Song Y.S., Kim H., Ku B.D., Lee W.W. Alteration of tremor dominant and postural instability gait difficulty subtypes during the progression of Parkinson's disease: analysis of the PPMI cohort. Front. Neurol. 2019;10:471. doi: 10.3389/fneur.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Coelln R., Gruber-Baldini A.L., Reich S.G., Armstrong M.J., Savitt J.M., Shulman L.M. The inconsistency and instability of Parkinson's disease motor subtypes. Parkinsonism Relat. Disord. 2021;88:13–18. doi: 10.1016/j.parkreldis.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Kang G.A., Bronstein J.M., Masterman D.L., Redelings M., Crum J.A., Ritz B. Clinical characteristics in early Parkinson's disease in a central California population-based study. Move. Disord. 2005;20(9):1133–1142. doi: 10.1002/mds.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.