Abstract
Left ventricular (LV) pseudoaneurysms are a rare disease entity associated with a multitude of etiologies. We describe the radiographic findings of an LV pseudoaneurysm arising as a complication of a leaking left ventricular assist device (LVAD) closure device. Computed tomographic angiography (CTA) imaging demonstrated an apical LV wall defect with a preperitoneal collection of extravasated contrast. A review of the patient's surgical history revealed prior LVAD placement and explant with placement of an LV closure device. Familiarity with the radiologic manifestation of LV pseudoaneurysms is critical to establish a prompt diagnosis and facilitate timely therapeutic intervention.
Keywords: Angiography, Computed tomography, Left ventricular assist device, Pseudoanuerysm
Introduction
As management strategies for heart failure continue to advance, LVADs are increasingly being utilized as destination or bridge therapy. A myriad of complications associated with LVADs are discussed in the literature such as inflow and outflow cannula complications, postoperative hemorrhage, thrombus formation, and infection [1]. In general, LV pseudoaneurysms are rare and have a high mortality. The majority of these pseudoaneurysms arise as sequelae of myocardial infarction whereas approximately 33% arise as a complication of cardiac surgery [2]. The literature describes a few cases of LVAD-associated pseudoaneurysms limited to the ascending aorta. Other reports describe LVAD-associated LV rupture secondary to catastrophic device-associated infection. LV pseudoaneurysms are prone to spontaneous rupture and many patients succumb to subsequent fatal cardiac tamponade and sudden cardiac death before achieving an imaging-based diagnosis. This tendency to rupture underscores the need for early diagnosis and prompt surgical repair. To our knowledge, this is one of a few cases that describes the CTA findings of a LV pseudoaneurysm arising as a complication of a LVAD explant secondary to displaced LV closure device (Ampltazer Plug) (Figure 1).
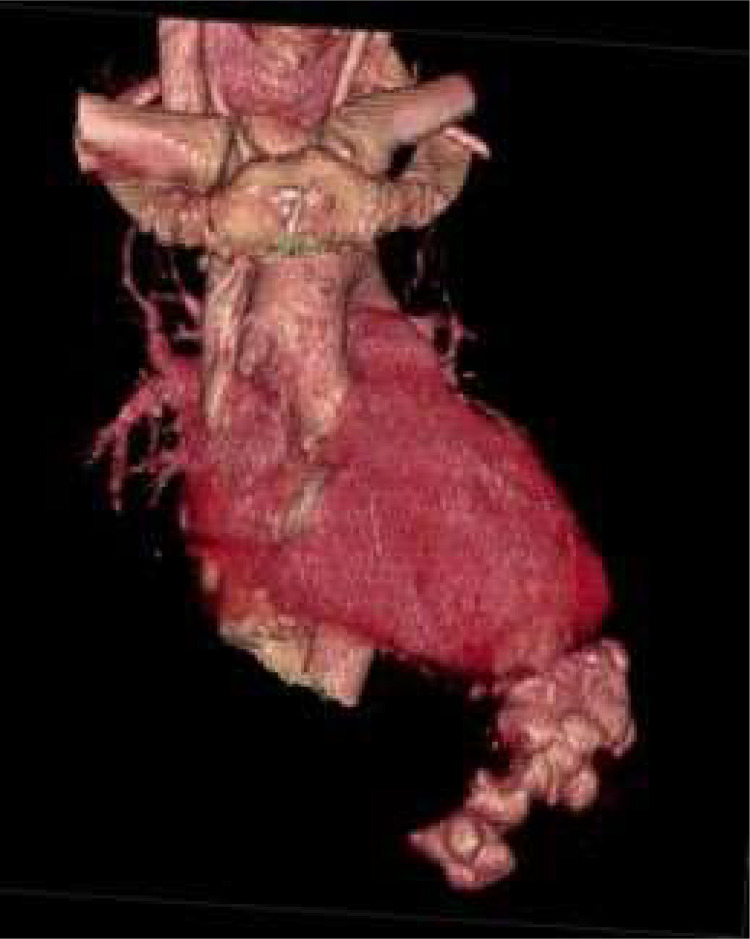
Fig 1.
3D rendered image of the heart depicting the LV pseudoaneurysm with extravasated contrast. In addition, there is a saccular outpouching of the anterolateral aspect of the ascending aorta which represents the site of prior aortotomy; where the outflow cannula of the LVAD was anastomosed to the aorta. Also, note the tricuspid annuloplasty that was performed at the time of LVAD implantation.
Patient information
A 46-year-old male presents to the emergency department with a 2-day history of progressive left-sided abdominal pain. His vital signs upon presentation were unimpressive, with a blood pressure of 124/82 mm Hg, heart rate of 84 bpm, temperature of 98.7°F, and a respiratory rate of 20 breaths per minute. Upon physical examination, a tender and pulsatile mass was palpated in the left upper quadrant. Past medical history was pertinent for aortic regurgitation leading to nonischemic dilated cardiomyopathy and heart failure with a reduced ejection fraction (EF) of 10%, requiring multiple cardiothoracic interventions and eventual LVAD placement. The patient underwent surgical implantation of a HeartMate-II LVAD and closure of the aortic valve using a pledged suture. After improvement in his EF to 45%, he underwent LVAD explant in 2015. The explantation was complicated by insufficiency and tearing of the LV wall, eventually necessitating repair with an Amplatzer plug. The patient had an uneventful recovery and was discharged on systemic anticoagulation and medical optimization of congestive heart failure with outpatient follow-up until presentation.
Timeline
At 8:33 AM an ECG was obtained that showed voltage criteria for LV hypertrophy, T-wave abnormality, and a prolonged QT interval. The symptoms and physical exam findings raised suspicion for acute aortic syndrome. After a discussion with the radiologist at 8:55 AM a CTA of the aorta was ordered. At 9 AM labs resulted and were significant for creatinine of 1.28 mg/dL, alkaline phosphatase of 317 IU/L, and a total bilirubin of 2.3 mg/dL. The cardiac troponin levels were 0.103 ng/mL. Additionally, the patient had leukocytosis of 11.8×109 WBCs/L and anemia with a hemoglobin of 9.6 g/dL.
At 9:18 AM the patient was brought to the CT scanner and by 9:36 AM findings were communicated by the radiologist; a contained rupture of the LV with the collection extending into a preperitoneal pocket compressing the left hepatic lobe and diaphragm (Figure 2). At the time, the report suggested that the image findings represented complications of a LV aneurysm repair or a possible cardiac pseudoaneurysm as a sequela of remote myocardial ischemia. The patient was subsequently transferred to a tertiary care institution at 10:45 AM for further management and surgical repair.
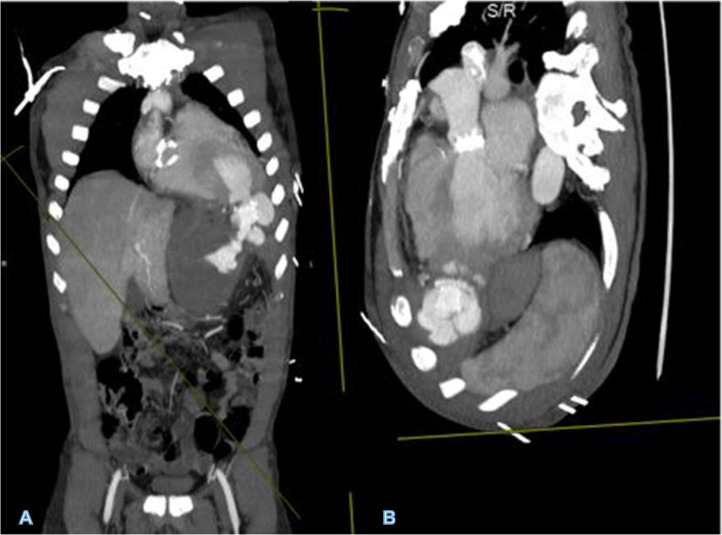
Fig 2.
MIP of original CT angiographic data set in coronal (A) and oblique (B) planes depicting all sites of LVAD implantation, outpouching at the ascending aorta representing outflow cannulation of the LVAD and LV apical defect from inflow cannula with Ampltazer plug and contrast leaking around it into the pseudoaneurysm. In addition, the aortic valve mechanical replacement can be seen in B. Incidentally noted tricuspid annuloplasty in image A.
Diagnostic assessment
There are many diagnostic challenges that radiologists are faced with, particularly in an emergency setting, that can impact patient management. Time sensitivity is critical in emergencies and any delays in radiological interpretation can lead to delayed diagnosis and treatment, which can worsen a patient's condition. Limited clinical information and a wide range of cases can increase the risk of misdiagnosis, potentially resulting in inappropriate treatments or interventions. Inaccurate or incomplete radiological findings can lead to suboptimal treatment decisions, impacting patient outcomes.
Our patient had a complex history of cardiothoracic surgical interventions at an external institution. These records were not available at presentation, nor were they obtainable within a consequential time frame. Furthermore, the imaging and reporting of highly specialized surgical cases, as well as familiarity with an ever-expanding roster of medical and surgical devices are challenging tasks. In this case, CTA imaging of the chest demonstrated cardiomegaly with an apical LV wall defect and a preperitoneal collection of contrast suggestive of an LV aneurysm or pseudoaneurysm (Figure 3).
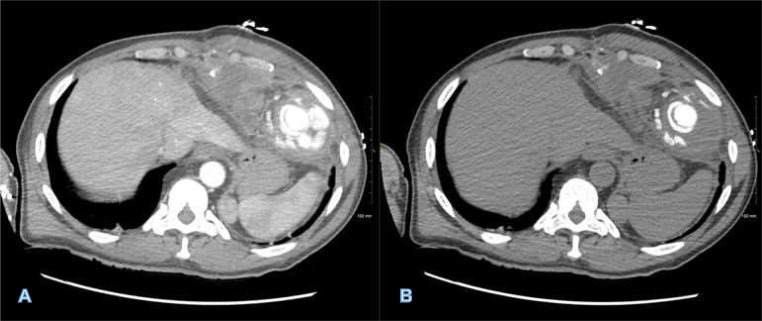
Fig 3.
CTA imaging of the contained pseudoaneurysm in soft tissue (A) and bone (B) windows. Image highlights the significant mass effect that preperitoneal collection exerts upon the liver and other intraperitoneal structures. In addition, the bone window highlights and distinguishes the Ampltazer plug from high attenuation contrast within the pseudoaneurysm which is contained by pericardium and fibrotic scar tissue.
Compared to true ventricular aneurysms, pseudoaneurysms may demonstrate a narrow neck at their origin from the ventricular wall [2,3] (Figure 4). Findings may also be accompanied by hemopericardium on cross-sectional and dynamic imaging [2], [3], [4], [5], [6] as was demonstrated in our case. Practically, however, distinguishing true aneurysms from false aneurysms may prove difficult, and surgical evaluation remains the gold standard for diagnosis [3].
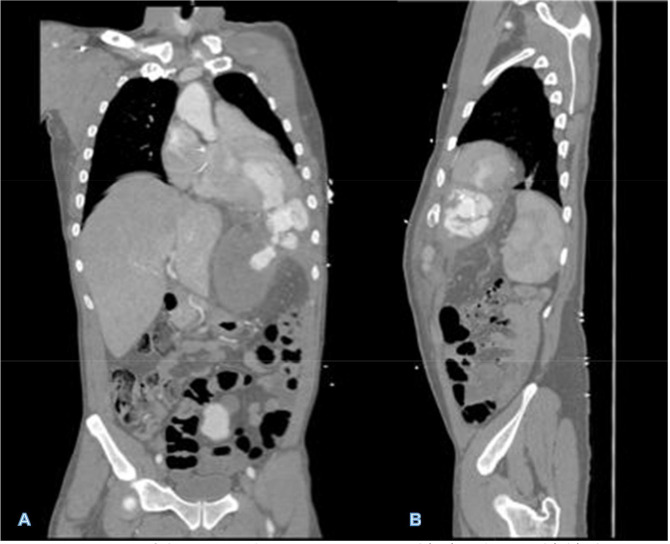
Fig 4.
CTA imaging of the LV pseudoaneurysm in coronal (A) and sagittal (B) planes. Note the narrow neck at the site of ventricular wall defect and hemopericardium corroborated by the presenting pulsatile left upper quadrant mass.
Therapeutic intervention
Upon transfer to an outside tertiary care facility, the patient underwent percutaneous closure of the defect. Given the size of the pseudoaneurysm and the diameter of the neck, 2 Amplatzer plugs were utilized for closure. Four days later, the patient underwent cardiopulmonary bypass, left anterior thoracotomy with removal of a portion of the sixth rib, removal of the apical connector from previous LVAD implant, removal of Amplatzer plugs, drainage of large preperitoneal fluid collection, and closure of LV apex to repair the pseudoaneurysm.
Follow-up and outcomes
The hospital course was complicated by staphylococcus epidermidis bacteremia. The patient was discharged home on postoperative day 7 after optimization of their congestive heart failure medications with instructions to follow up on an outpatient basis. Patient is now 2 months postoperation and reports feeling well.
Discussion
A pseudoaneurysm typically represents a contained rupture of a blood vessel or myocardial wall, circumscribed by the pericardium, scar tissue, and adhesions or thrombus [3]. The LV is the most common location for cardiac pseudoaneurysms, which may arise as sequelae of myocardial ischemia, trauma, infection, or cardiac surgery [3]. When the LV is implicated, the posterolateral, anterior, and apical mural segments are frequently involved [4]. In contrast, a true aneurysm usually results from weakening and dilatation of the ventricular wall involving all the myocardial layers including the endocardium, myocardium, and pericardium. Myocardial thinning facilitates bulging and paradoxical movement of the affected chamber during the cardiac cycle, predisposing to rupture [3,7]. This is particularly true in the early postinfarction period. However, after fibrosis and scarring take place rupture is exceedingly rare [8]. In contradistinction, LV pseudoaneurysms may rupture at any time with a significantly increased propensity for rupture. Thus, differentiating these entities is important as the risk of rupture of pseudoaneurysms is approximately 30%-45% within the first year [9]. Early surgical intervention is recommended despite a postoperative mortality rate of 20% as the risk of fatal rupture outweighs the risks of surgery [10].
Presenting symptoms may include congestive heart failure, dyspnea, and chest pain. However, a significant number of patients are asymptomatic [11]. Clinical examination may demonstrate a cardiac murmur or displaced apical impulse, with ST-segment ECG changes, however, these findings are neither specific nor sensitive, and imaging is usually required for accurate diagnosis [2,3]. Initial evaluation may be obtained with transthoracic echocardiography, however invasive and CT angiography provide a much higher diagnostic yield, with cardiac MRI and transesophageal echocardiography serving as alternative modalities for diagnosis [3].
On imaging, it is crucial to identify the integrity and continuity of the myocardium. An abrupt transition from normal contour to a myocardial outpouching with a narrow neck and myocardial discontinuity is suggestive of an LV pseudoaneurysm. In contrast, maintained myocardial integrity, compromising the endocardium, myocardium, and pericardium, with a mural outpouching containing a wide neck of transition are imaging features suggestive of a true aneurysm. Mural thrombosis within the aneurysmal sac is a feature common to both entities. Findings may also be accompanied by hemopericardium on cross-sectional and dynamic imaging [2], [3], [4], [5], [6].
Due to the ambiguous presentation and nonspecific physical exam findings, a high clinical suspicion and early utilization of diagnostic imaging can be lifesaving. Our case highlights the direct impact that prompt imaging had on our patient; who ultimately had a favorable outcome despite the grave imaging findings and potentially lethal disease entity they represented. Thus, accurate and timely diagnosis are crucial in cases of LV pseudoaneurysms owing to their high mortality risk [2,7].
Patient consent
I, Mohanad Kurdi, declare and confirm that written and informed consent for publication of this case was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Carr CM, Jacob J, Park SJ, Karon BL, Williamson EE, Araoz PA. CT of left ventricular assist devices. Radiographics. 2010;30(2):429–444. doi: 10.1148/rg.302095734. [DOI] [PubMed] [Google Scholar]
- 2.Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32(3):557–561. doi: 10.1016/s0735-1097(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 3.Hulten EA, Blankstein R. Pseudoaneurysms of the heart. Circulation. 2012;125(15):1920–1925. doi: 10.1161/CIRCULATIONAHA.111.043984. [DOI] [PubMed] [Google Scholar]
- 4.Tuan J, Kaivani F, Fewins H. Left ventricular pseudoaneurysm. Eur J Echocardiogr. 2008;9(1):107–109. doi: 10.1016/j.euje.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm. A review of the literature. Chest. 1997;111(5):1403–1409. doi: 10.1378/chest.111.5.1403. [DOI] [PubMed] [Google Scholar]
- 6.Zoffoli G, Mangino D, Venturini A, et al. Diagnosing left ventricular aneurysm from pseudo-aneurysm: a case report and a review in literature. J Cardiothorac Surg. 2009;4:11. doi: 10.1186/1749-8090-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin AC, Sandoval E, Letsou GV, Mallidi HR, Cohn WE, Frazier OH. Surgical approach to continuous-flow left ventricular assist device explantation: A comparison of outcomes. J Thorac Cardiovasc Surg. 2016;151(1):192–198. doi: 10.1016/j.jtcvs.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Vlodaver Z, Coe JI, Edwards JE. True and false left ventricular aneurysms. Propensity for the alter to rupture. Circulation. 1975;51:567–572. doi: 10.1161/01.cir.51.3.567. [DOI] [PubMed] [Google Scholar]
- 9.Alapati L, Chitwood WR, Cahill J, Mehra S, Movahed A. Left ventricular pseudoaneurysm: a case report and review of the literature. World J Clin Cases. 2014;2(4):90–93. doi: 10.12998/wjcc.v2.i4.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prêtre R, Linka A, Jenni R, Turina MI. Surgical treatment of acquired left ventricular pseudoaneurysms. Ann Thorac Surg. 2000;70(2):553–557. doi: 10.1016/s0003-4975(00)01412-0. [DOI] [PubMed] [Google Scholar]
- 11.Yeo TC, Malouf JF, Oh JK, Seward JB. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med. 1998;128(4):299–305. doi: 10.7326/0003-4819-128-4-199802150-00010. [DOI] [PubMed] [Google Scholar]