Inhibition of 2OG human oxygenases by selected thiazole and N-hydroxythiazole analogues.
| Entry | Cmpda |

|
IC50 [μM]b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | FIHc | PHD2d | AspHe | KDM4Af | JMJD5g | ||
| i | BNSh | OH |

|
H | 0.30 ± 0.07 | 0.11 ± 0.00 | 3.4 ± 0.1 | 67.4 ± 39.8 | 0.25 ± 0.01 |
| ii | 4 | OH |
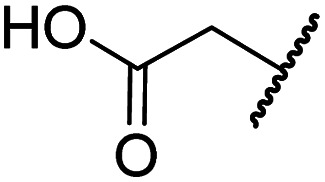
|
H | 0.28 ± 0.00 | 0.50 ± 0.05 | 9.6 ± 1.3 | >100 | 0.48 ± 0.01 |
| iii | 5 | H |

|
H | >100 | >100 | >100 | >100 | >100 |
| iv | 6 | OH |

|
H | 61.0 ± 3.1 | 5.2 ± 2.6 | 25.6 ± 12.7 | >100 | 60.5 ± 1.0 |
| v | 7 | OH |

|
Me | 36.7 ± 4.9 | 3.1 ± 1.4 | 24.3 ± 12.3 | >100 | >100 |
| vi | 8 | OH | H | H | >100 | >100 | >100 | >100 | >100 |
| vii | 9 | OH |
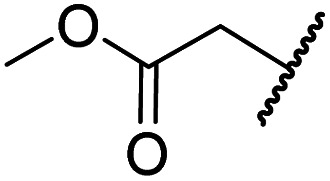
|
H | >100 | >100 | 96.7 ± 3.2 | >100 | >100 |
| viii | 3 | OH |

|
H | >100 | >100 | 68.0 ± 26.8 | >100 | >100 |
| ix | 10 | OH |

|
H | >100 | >100 | 43.6 ± 3.7 | >100 | 77.5 ± 23.8 |
| x | 11 | OH |

|
H | >100 | >100 | >100 | >100 | >100 |
| xi | 12 | OH |

|
H | >100 | >100 | 83.3 ± 6.7 | >100 | >100 |
| xii | 13 | OH |

|
H | 2.2 ± 0.6 | 3.0 ± 1.3 | 47.3 ± 4.7 | >100 | >100 |
All chiral N-hydroxythiazole derivatives were prepared as racemic mixtures.
Mean average ± SD of two independent experiments (each composed of technical duplicates).
Using 0.15 μM FIH, 10.0 μM 2OG and 5.0 μM HIF-1α C-TAD788–822.61
Using 0.15 μM PHD2181–426, 10.0 μM 2OG and 5.0 μM HIF-1α CODD556–574.61
Using 0.05 μM His6-AspH315–758, 3.0 μM 2OG and 1.0 μM hFX-CP101–119.58
Using 0.15 μM KDM4A, 10.0 μM 2OG and 10.0 μM H31–15K9me31–15.69
Using 0.15 μM JMJD5, 2.0 μM 2OG and 2.0 μM RSP6128–148.70
BNS has a 2-naphthylmethyl sulfonyl group rather than a phenyl sulfonyl group. Inhibition assays were performed using SPE-MS as described in the ESI.
