Fig. 1. Importance of pyridine halogenation reactions and a distinct strategy based on ring-opened intermediates.
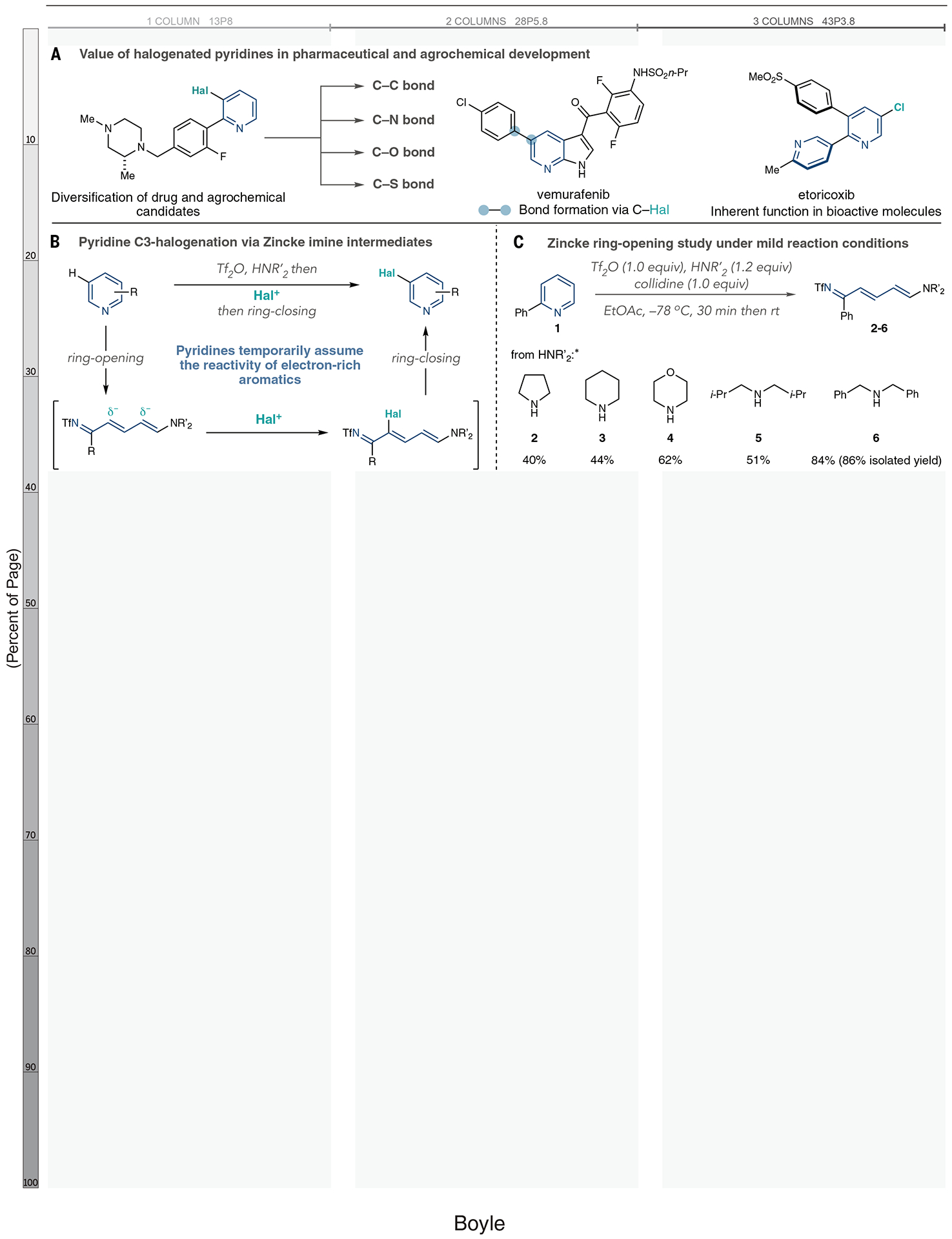
(A) Examples of pyridine halogenation products and derivatives in drug and agrochemical development. (B) A ring-opening, halogenation, ring-closing synthetic strategy. (C) Ring-opening study using amine nucleophiles. *Yields calculated from integrated 1H NMR spectra of the crude reaction mixture using triphenylmethane as an internal standard. R, general organic group; Me, methyl; Hal, halogen; Tf, trifluoromethylsulfonyl; collidine, 2,4,6-trimethylpyridine; Et, ethyl; Ac, acetate; Ph, phenyl; i-Pr, isopropyl; rt, room temperature.
