FIGURE 4.
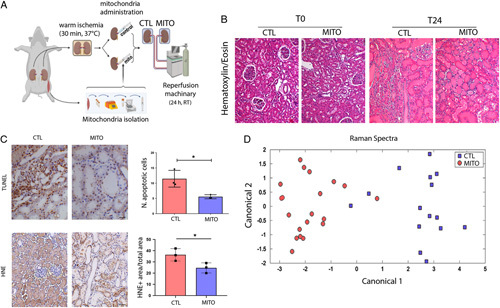
MITO improves IRI in a model of donation after cardiac death (DCD) renal transplant. A, An ex vivo model of DCD kidneys was used to test the hypothesis that mitochondrial transplantation (MITO) improves the pathophysiology associated with IRI. Kidneys were subjected to warm ischemia for 30 minutes, followed by injection with vehicle (CTL) or mitochondria isolated from 1g of autologous psoas muscle (MITO). After 30 min, kidneys were perfused for 24 hours. (n=4 animals, 5 kidneys (one CTL discarded), 2 experimental groups, CTL and MITO). B, The presence of macroscopic damage in control (CTL) and mitochondrial treated (MITO) kidneys was shown by hematoxylin and eosin staining. C, thymidine deoxyribose-mediated deoxy-UTP nick end labeling (TUNEL) assay and 4-hydroxy-2-nonenal (HNE) showed a decrease of apoptotic cells and lipid peroxidation, respectively, in MITO kidneys (representative images) that was confirmed by quantification. Student t test was performed: *P < 0.05. D, Raman spectra, collected at sequential timepoints from control and MITO kidneys were baseline normalized and then analyzed using principal component analysis and discriminate analysis of principal components. The canonical plot matrix shows differences in the molecular composition of perfusate from control and MITO kidneys appearing as definitive separated clusters. MITO treated kidneys shed fewer molecules/molecular species over 24 hours than CTL kidneys, indicating greater stability/viability [shown statistically by calculation of Total Spectral Distance vs Analytical Standard (Surine) using the RametrixTM Toolboxes]. CTL indicates control.
