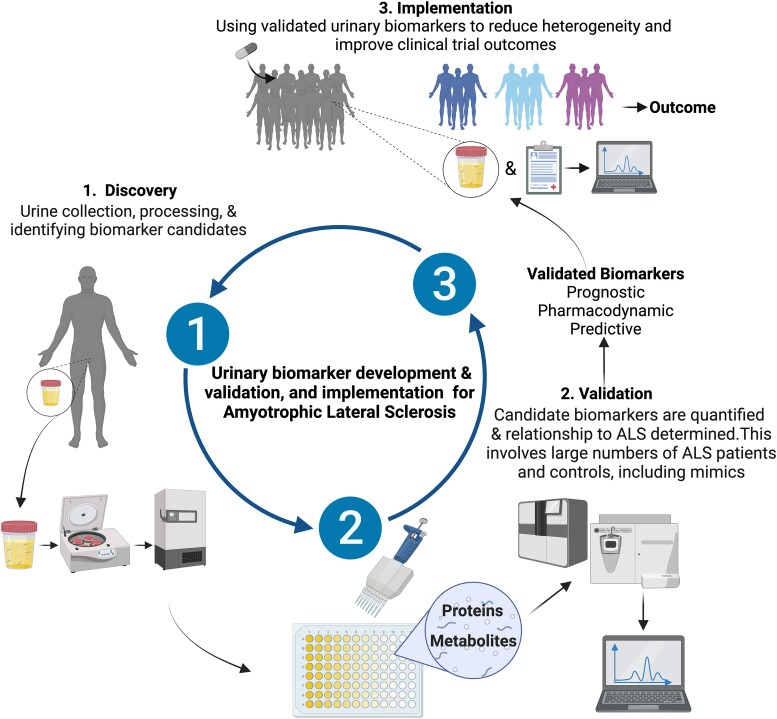
Figure 2.
Urinary biomarker development and implementation for ALS. The urinary biomarker development includes discovery, validation and utilization. Urine samples are collected from people with ALS and controls for discovery-type experiments. Biomarkers are isolated, measured and compared with clinical characteristics and pathology to determine if a possible candidate biomarker. The biomarkers are then validated across larger cohorts of ALS and controls, including mimic diseases. Validated prognostic, pharmacodynamic and predictive biomarkers can then be utilized to reduce heterogeneity and determine efficacy in clinical trials and determine outcomes (drug was a success or not).