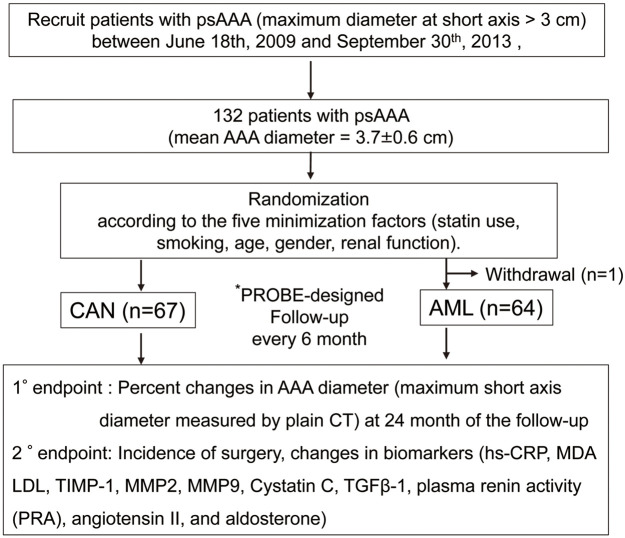
Figure 1.
Study overview. *PROBE: prospective randomized open blinded end-point design. 1°, primary; 2°, secondary; AAA, abdominal aortic aneurysm; AML, amlodipine; CAN, candesartan; CT, computed tomography; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MDA, malondialdehyde; MMP, matrix metalloproteinase; PRA, plasma renin activity; psAAA, abdominal aortic aneurysm at the presurgical stage; TGF-β1, transforming growth factor-β1; TIMP1, tissue-specific inhibitor of metalloproteinase 1.