Figure 4. Assessing nhpSer encoding into sfGFP by Phos-tag gel electrophoresis.
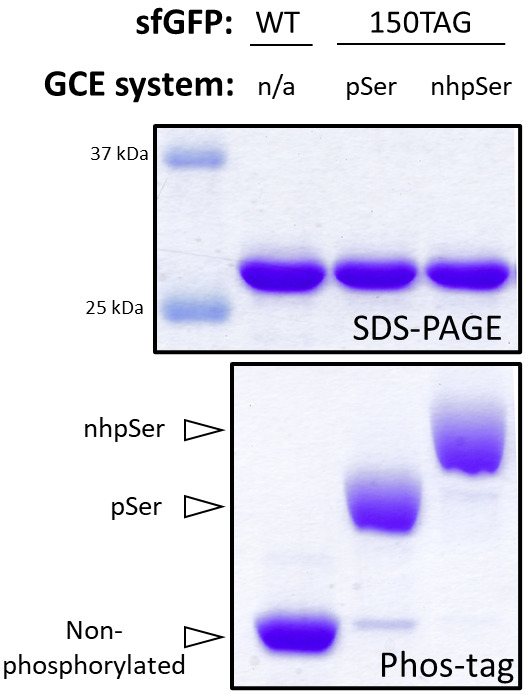
SDS-PAGE (top) and Phos-tag (bottom) gels of purified WT sfGFP, sfGFP-150pSer, and sfGFP-150nhpSer proteins. The sfGFP-150pSer protein was produced following our prior protocol (Zhu et al., 2022) and the sfGFP-150nhpSer protein was produced using the protocol described here. Because they migrate differently on Phos-tag gels, non-phosphorylated, pSer-containing, and nhpSer-containing proteins can be resolved from one another, and the fraction of each form can be easily evaluated in each sample. In this example, the sfGFP-150pSer protein sample is > 90% phosphorylated with pSer, with only trace amounts of non-phosphorylated protein that migrates identically as the wild-type sfGFP protein, while the sfGFP-150nhpSer protein is > 95% phosphorylated with nhpSer, with only trace amounts of pSer-containing contaminant protein and no detectable non-phosphorylated protein. Note that all proteins migrate identically on SDS-PAGE, indicating the electrophoretic shifts observed in Phos-tag are due to phosphorylation status and not differences in protein size. These samples were incubated at 95 °C for 5 min in standard Laemmli buffer prior to their loading on gel. The Phos-tag gel contained 50 μM Phos-tag acrylamide, 100 μM MnCl2, and a 29:1 acrylamide:bis-acrylamide ratio and was run at 120 V for 1.5 h at room temperature.
