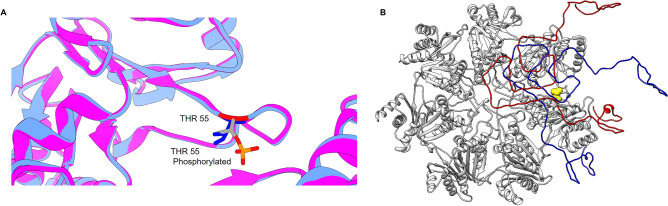
Figure 6.
Phosphorylation of Thr55 does not change local conformation but may affect the interaction between α5-subunit and proteins. (A) Representation of part of the three-dimensional structure of the 20S proteasome (pdb 3D29) focused on the region where the Thr55 residue susceptible to phosphorylation is located. The structure with dephosphorylated Thr55 is shown in pink while the simulated structure with phospho-Thr55 in represented in blue. (B) Representation of models of interaction between the 20S proteasome α-ring and α-synuclein. The models were generated with the Haddock program. Interaction of α-synuclein with the proteasome α-ring in the absence or presence of Thr55 phosphorylation is represented in blue and red, respectively. Phosphorylated threonine 55 is shown in yellow.