Abstract
Spatial omics has emerged as a rapidly growing and fruitful field with hundreds of publications presenting novel methods for obtaining spatially resolved information for any omics data type on spatial scales ranging from subcellular to organismal. From a technology development perspective, spatial omics is a highly interdisciplinary field that integrates imaging and omics, spatial and molecular analyses, sequencing and mass spectrometry, and image analysis and bioinformatics. The emergence of this field has not only opened a window into spatial biology, but also created multiple novel opportunities, questions, and challenges for method developers. Here, we provide the perspective of technology developers on what makes the spatial omics field unique. After providing a brief overview of the state of the art, we discuss technological enablers and challenges and present our vision about the future applications and impact of this melting pot.
Keywords: genomics, metabolomics, proteomics, spatial omics, transcriptomics
Subject Categories: Chromatin, Transcription & Genomics; Methods & Resources; Proteomics
This Review discusses the spatial omics field from the point of view of technology developers. It provides an overview of the state of the art, discusses technological enablers and challenges and presents a vision about the future applications and impact of spatial omics technologies.
Introduction
Biology is driven by interactions of molecules and cells with each other. Gaining insights into the intricate yet robust spatial regulation of biological processes, at the scales of organelles, cells, tissues, organs, and organisms, is key to understanding fundamental mechanisms of life. Furthermore, understanding the spatial context of the alterations of molecular and cellular programs during disease in situ, in toto, and in vivo is critical for diagnostics, developing novel and safe drugs, and discovering therapies for currently uncurable diseases.
The field of omics holds great potential to provide information‐rich readouts of molecular programs across all levels of the central dogma. It is currently undergoing an explosion with respect to spatial methods development, with the most prominent example being spatial transcriptomics, which was recognized as “the method of the year 2020” (Larsson et al, 2021; Marx, 2021; Zhuang, 2021). This rapid technological progress is notable across various omics modalities: genomics, epigenomics, proteomics, metabolomics, and fluxomics. The emerging field of spatial omics is now at a tipping point as the technology steps outside the technology developers' laboratories and spreads rapidly and widely, finding many applications in biology and molecular medicine.
In this Perspective, we discuss the origins and developments, the current drivers and challenges as well as the future potential of spatial omics from a viewpoint of experimental and computational technology developers. We do not aim to provide a comprehensive review of the state of the art of technologies and approaches in the field, for which we point the reader to recent excellent reviews (Rao et al, 2021; Moffitt et al, 2022; Moses & Pachter, 2022; Palla et al, 2022a; Vandereyken et al, 2023), but rather to highlight unique interdisciplinary aspects of the field and stimulate further cross‐fertilization.
Where do spatial omics stand? An overview of the available technologies
Spatial omics methods are evolving at the intersection of two emerging directions: (i) in situ approaches that build on highly multiplex fluorescence microscopy or imaging mass spectrometry and (ii) ex situ approaches based on either next‐generation sequencing or mass spectrometry (Fig 1).
Figure 1. Evolutionary path of spatial omics technologies: unraveling the convergence.
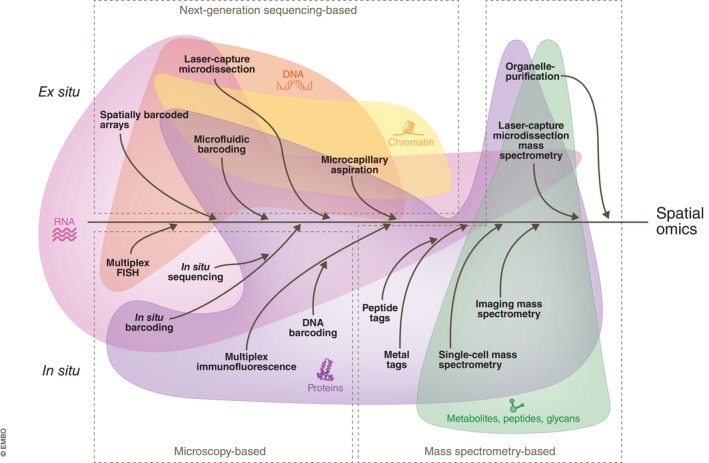
Core capabilities stemming from fields of traditional omics, microscopy and mass spectrometry have led to emergence of diverse methods for spatial omics. Initially focused on only one molecular component, many of these approaches are increasingly getting combined to expanded repertoire of methods into multi‐omics domains.
In situ approaches
Transcriptomics, proteomics, genomics, epigenomics using highly multiplex fluorescence microscopy
Fluorescence microscopy has been for decades a go‐to approach in cell biology, for observing target biomolecules with high resolution in live and fixed cells. Fluorescence can be used for spatial mapping of gene expression (e.g., by RNA‐FISH) as well as localization of proteins (e.g., by immunofluorescence) and metabolites (e.g., using fluorescent metabolite sensors) in subcellular compartments, cells, tissues, and even whole organisms. The wish to increase the number of targeted molecules has been clashing with the limits of the spectral overlap. This limitation led to exploring various methods of in situ labeling to increase multiplexing, including: (i) hyperspectral imaging that utilizes a broader spectrum from fluorescence to Raman signatures (Wei et al, 2017; Du et al, 2020) particularly for spatial metabolomics or spectral unmixing in which spectral overlaps can be minimized or are resolved computationally (Gerner et al, 2012; Mehta et al, 2018; Seo et al, 2022; Lin et al, 2023), or (ii) cyclic labeling or cyclic imaging. For protein detection, traditional immunofluorescence methods were expanded by applying antibodies in cycles of staining and bleaching/elution (Gerdes et al, 2013; Lin et al, 2015, 2018; Gut et al, 2018). Furthermore, the use of nucleic acids offered multiplexing options through orthogonal DNA barcodes (Schueder et al, 2020), allowing simultaneous application of many antibodies and fast detection cycles with programmed hybridization/dehybridization of fluorescent oligos (Wang et al, 2012, 2017; Jungmann et al, 2014; Agasti et al, 2017; Schueder et al, 2017; Saka et al, 2019; Schürch et al, 2020).
These probe–erase–relabel approaches were better suited for linearly increasing the multiplexed detection of proteins, which show complex and overlapping spatial localizations and high dynamic range of expression. On the contrary, the more discrete spotty localization of RNAs allowed exponentially increasing the multiplicity of target detection through combinatorial barcoding. Combining RNA‐FISH with similar iterative detection cycles facilitated scaling multiplexing to 1000s of targets with methods such as SeqFISH and MERFISH (Lubeck et al, 2014; Chen et al, 2015; Moffitt et al, 2016; Eng et al, 2019; Xia et al, 2019b), and performing various approaches of cyclic decoding or in situ sequencing (Ke et al, 2013; Lee et al, 2014, 2022; Wang et al, 2018b; Gyllborg et al, 2020; Sountoulidis et al, 2020; Borm et al, 2023). Similar linear and exponential strategies were also applied in combination with DNA‐FISH to reveal new insights into spatial organization of chromatin and nuclear architecture (Beliveau et al, 2015; Wang et al, 2016; Nir et al, 2018; Mateo et al, 2019; Nguyen et al, 2020; Su et al, 2020; Takei et al, 2021b, 2021a, preprint: Takei et al, 2023). Moreover, incorporating combinatorial barcoding into a CUT&TAG‐like strategy (Bartlett et al, 2021), where epigenetic modifications of interest are labeled with antibodies and the associated genomics loci are detected by MERFISH after in situ transcription, allowed the spatial epigenomic profiling of hundreds of enhancers and promoters (Lu et al, 2022).
Proteomics, metabolomics, and lipidomics by using imaging mass spectrometry
In parallel, imaging mass spectrometry, where for a raster of pixels, a small amount of material from every pixel is desorbed, simultaneously ionized, and analyzed by mass spectrometry, has emerged as an indispensable and promising technology for in situ spatial mapping of proteins, peptides, metabolites, lipids, and glycans (Alexandrov, 2020). The most common approach in imaging mass spectrometry is matrix‐assisted laser desorption ionization (MALDI) imaging (Palmer et al, 2016). Numerous other approaches are available that do not use laser (Takáts et al, 2004). For spatial metabolomics applications, the current focus of imaging mass spectrometry is predominantly tissue cryosections, allowing for analyses of sections up to the size of a whole animal (Khatib‐Shahidi et al, 2006). The latest methods even demonstrate subcellular resolution (Niehaus et al, 2019; Pareek et al, 2020; Rovira‐Clavé et al, 2021). Untargeted detection of proteins in tissue sections with imaging mass spectrometry was demonstrated as early as 2001 (Stoeckli et al, 2001), with further improvements in resolution and speed over the years (Spraggins et al, 2016). Using imaging mass spectrometry in conjunction with metal‐tagged antibodies allowed reaching single‐cell level resolution for highly multiplexed targeted spatial proteomics (Angelo et al, 2014; Giesen et al, 2014). In parallel, using imaging mass spectrometry for metabolomics was boosted in the 2000s with the introduction of high‐resolution mass spectrometry analyzers. Imaging mass spectrometry is often used for detecting lipids due to the ease of sample preparation and detection of molecules of this class (Bowman et al, 2019). Recently, spatially resolved detection of N‐linked glycans attached to asparagine residues in proteins became possible, enabling spatial glycomics (McDowell et al, 2023). Imaging mass spectrometry was demonstrated to be an enabling method for spatial isotope tracing to spatially map metabolic activities as early as 2012 (Steinhauser et al, 2012; Louie et al, 2013). Later on, organismal‐scale fluxomics analyses using mass spectrometry revealed circulating lactate as an important energy source (Hui et al, 2017). And recently, imaging mass spectrometry was applied for high‐resolution spatial isotope tracing for cell‐type‐specific dynamics and metabolic activity in tissues (Wang et al, 2022a, 2022b).
Ex situ approaches
Spatially resolved sequencing
Ex situ spatial sequencing methods are increasing in both numbers and diversity. In transcriptomics, the emergence of single‐cell RNA sequencing through droplet‐based separation of individual cells created opportunities for profiling cells by next‐generation sequencing (Klein et al, 2015; Macosko et al, 2015). Yet, these analyses were inherently unable to capture the spatial context of cells and to directly investigate cell–cell interactions (although indirect methods have been proposed, Armingol et al, 2021). This limitation led to a parallel exploration of ways to link spatial information with the sequencing data that are obtained ex situ when analyzing tissue sections. Several approaches were developed such as capturing RNA molecules on spatially barcoded arrays (Ståhl et al, 2016; Rodriques et al, 2019; Vickovic et al, 2019; Cho et al, 2021; Fu et al, 2022; Chen et al, 2022b), constructing positional DNA barcodes in situ by microfluidic delivery (Dbit‐Seq, Liu et al, 2020), photocrosslinking (Light‐Seq, Kishi et al, 2022) or photouncaging of spatial index oligos (Zip‐Seq, Hu et al, 2020), spatially confined collection of biomolecules or probes by microregion sequencing using light‐based cleavage for Digital Spatial Profiling (GeoMx, Merritt et al, 2020), and microdissection (LCM‐Seq (Nichterwitz et al, 2016), Image‐Seq (Haase et al, 2022)), mechanical isolation (Pick‐Seq, Maliga et al, 2021), or even extracting RNA from living cells by fluid force microscopy (Chen et al, 2022a).
Although most of these methods were initially applied to spatial transcriptomics, the majority could also be leveraged for spatial proteomics by incorporating readouts for DNA‐barcoded antibody libraries. Examples include the studies by Vickovic et al (2022), Liu et al (2023) and Ben‐Chetrit et al (2023). A subset of these ex situ strategies have been combined with in situ transposition to incorporate sequencing adapters into fixed genomic DNA, using Tn5 transposase similarly to ATAC‐Seq (Buenrostro et al, 2013) so that native spatial positions of accessible genomic DNA are preserved, enabling studies of spatial (epi)genomics (Chen et al, 2016b; Payne et al, 2020; Deng et al, 2022; Mangiameli et al, 2023).
Particularly for spatial transcriptomics, the parallel development in situ imaging or ex situ sequencing‐based methods, and novel combinations of workflows in these domains was critical for the quick expansion and success of the field (Larsson et al, 2021; Zhuang, 2021). It minimized risks, cross‐stimulated developments, and helped engage research groups with diverse backgrounds ranging from DNA biotechnology, microfluidics, sequencing, and microscopy to image analysis, and bioinformatics. Recently, these two branches began converging. On one hand, the resolution and sensitivity of the sequencing‐derived approaches are catching up with imaging (Vickovic et al, 2019; Cho et al, 2021; Stickels et al, 2021; Fu et al, 2022; Chen et al, 2022b). On the other hand, the multiplexing depth and throughput of imaging gets closer to omics level, including the latest FISH‐based techniques that can image the expression of 1000s of genes (Eng et al, 2019; Su et al, 2020; Takei et al, 2021b, 2021a). We foresee the FISH‐like targeted probe design strategies getting increasingly used to improve the sensitivity of sequencing‐based approaches, as an alternative to the more standard polyA‐capture, especially for more challenging samples such as FFPE preparations (for example, 10X Genomics Visium FFPE kit or Xenium system, or Nanostring GeoMx or CosMx systems).
Ex situ mass spectrometry
Although, as previously discussed, mass spectrometry is currently more popular for in situ analyses by means of imaging mass spectrometry, there are several notable ex situ approaches. Among them, laser‐capture microdissection is commonly used for extracting individual cells or small regions of interest from a tissue section for subsequent mass spectrometry analysis, as demonstrated for spatial proteomics (Zhu et al, 2018a,b; Mund et al, 2022) and spatial metabolomics already as early as in 2005 (Schad et al, 2005). Another group of ex situ methods often referred as spatial metabolomics is the proteome profiling of purified organelles or other cell compartments followed by mass spectrometry (Dunkley et al, 2004). Such methods enabled creating a subcellular map of the human proteome (Mulvey et al, 2017; Thul et al, 2017) and led to a proximity‐based mapping of the proteome of a human cell (Go et al, 2021). Lately, metabolomics was also applied for purified organelles (Chen et al, 2016a; Zhu et al, 2021). On another, opposite end of the spatial scales, spatial sampling followed by mass spectrometry was used for creating 3D molecular cartography maps at the organismal and supra‐organismal scales (Bouslimani et al, 2015; Petras et al, 2017).
Spatial omics is empowered by bespoke computational methods
These technological developments have been accompanied by a rapid evolution of computational methods (Fig 2). On one hand, image processing and high‐dimensional analysis approaches have brought handling of highly multiplex fluorescence microscopy data closer to omics data. Image data typically offer single‐cell or subcellular resolution. Cell segmentation was substantially improved with the incorporation of powerful deep learning approaches focused on highly multiplexed imaging (Greenwald et al, 2021; Yapp et al, 2022) to further incorporate information about the localization of cells and their neighbors in the tissue. Alternatively, segmentation‐free methods provide a different approach for dealing with images where cells are hard to segment. These approaches can assign molecules to cells based on the likelihood of particular transcriptional compositions and cell morphology by incorporating prior knowledge of cell types obtained from scRNA‐seq (Park et al, 2021; Petukhov et al, 2021).
Figure 2. Unveiling new insights with spatial omics.
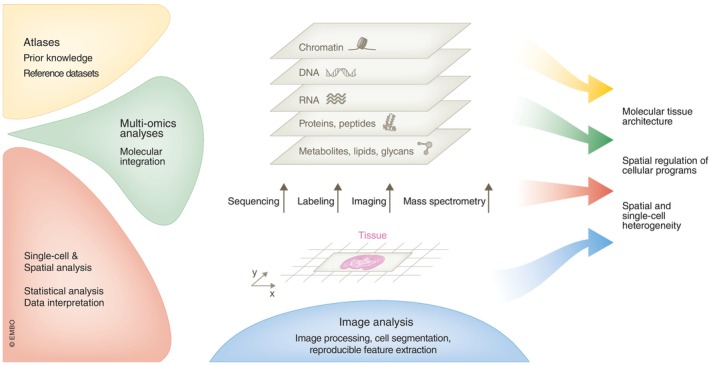
An overview of the data sources and knowledge inputs, computational approaches employed for data analysis, and pivotal questions central to spatial omics. The illustration outlines the individual omics layers, each providing a complementary view of the cellular programs.
Different methods have been developed that can identify spatial patterns in the expression of genes or correlations across genes, based on the resulting processed data (Edsgärd et al, 2018; Svensson et al, 2018; Ghazanfar et al, 2020; Sun et al, 2020). Numerous approaches for spatial data analyses have been developed, focused on different aspects: revealing interactions between the markers across different spatial contexts, investigating the distribution of identified cell types in the neighborhood of each cell (Schapiro et al, 2017; Goltsev et al, 2018; Keren et al, 2018), or decomposing the effect among markers at the cellular intrinsic, extrinsic, and intercellular levels (Arnol et al, 2019; Tanevski et al, 2022). Another emerging direction is the computational integration with single‐cell omics data. In transcriptomics, this helped tackle some limitations of spatial transcriptomics: first, by deconvolving cosampled cell types in spatially resolved sequencing with spot sizes encompassing 5–100 cells (Andersson et al, 2020; preprint: Kleshchevnikov et al, 2020; Cable et al, 2021; Elosua‐Bayes et al, 2021; Li et al, 2022) and second, by imputing unmeasured transcripts in targeted multiplex measurements (Biancalani et al, 2021; preprint: Rahimi et al, 2023). In parallel, computational approaches can estimate the location of single cells dissociated from the tissue (Bageritz et al, 2019; Nitzan et al, 2019; preprint: Biancalani et al, 2020) or map them via fiducial genes (Achim et al, 2015; Satija et al, 2015; Tanevski et al, 2020). Furthermore, methods have been developed to identify spatial domains with common molecular profiles and neighborhood structures (Hu et al, 2021; Zhao et al, 2021), and to study cell–cell interactions (Cang & Nie, 2020; Yuan & Bar‐Joseph, 2020; Garcia‐Alonso et al, 2022; Tanevski et al, 2022; Cang et al, 2023; Fischer et al, 2023).
Several software frameworks are available for analyzing spatially resolved data, such as Squidpy (Palla et al, 2022b), SpatialExperiment (Righelli et al, 2022), Giotto (Dries et al, 2021), and Seurat (preprint: Hao et al, 2022), which are primarily focused on spatial transcriptomics data, and MCMICRO (Schapiro et al, 2022a) for multiplexed protein data. These frameworks, which are continuously evolving, provide integrated suits that facilitate the application of state of the art methods (Heumos et al, 2023) for users without advanced computational skills.
Enablers and challenges
Looking back at the recent history of spatial omics from our point of view as technology developers, we asked what were the key enablers that jump‐started technological developments in spatial omics at the fast pace, that was key for establishing this field and for contributing to its success.
Pushing the technical limitations
When spatial omics started emerging, cyclic imaging paved the way to deepen the information content of microscopy experiments. The exploration and development of protocols that go beyond conventional fluorescent staining and imaging, and allow reprobing the same sample were crucial for imaging‐based multiplexing both for protein and RNA detection. For proteins, the use of direct primary antibody conjugations with fluorophores and alternative tags such as metals or DNA facilitated the creation of bigger panels. This also created a demand for sourcing of primary antibodies in formats that are more amenable to direct conjugation and creating kits for increasing the accessibility of the different protocols, as well as a higher scrutiny for antibody qualifications (Hickey et al, 2021).
For RNA and DNA detection, easy and cheaper access to synthetic oligos and the development of high‐fidelity FISH protocols utilizing probe tiling as in single molecule FISH (Femino et al, 1998; Orjalo et al, 2011; Beliveau et al, 2012) was important to attain a good signal‐to‐noise ratio while offering flexibility with probe design. Other in situ amplification approaches that have been transforming in situ RNA, DNA, and protein labeling include reactive probe deposition (Kerstens et al, 1995), DNA branching (Player et al, 2001; Wang et al, 2012; Kishi et al, 2019; Saka et al, 2019; Xia et al, 2019a), in situ polymerization (by rolling circle amplification (Lizardi et al, 1998; Lee et al, 2014; Nagendran & Riordan, 2018; Wang et al, 2018b; Liu et al, 2021), hybridization chain reaction (Dirks & Pierce, 2004; Choi et al, 2010, 2014, 2018; Shah et al, 2016; preprint: Wang et al, 2020)), or chemical ligation (Rouhanifard et al, 2018; Dardani et al, 2022).
Technical limitations in resolution, sensitivity, and throughput still constitute important barriers. In addition to signal amplification, the integration of super‐resolution (Jacquemet et al, 2020) and expansion microscopy approaches (Wen et al, 2023) with spatial omics methods start to provide valuable improvements in resolution and sensitivity for imaging‐based spatial omics (for example (Shah et al, 2017; Nir et al, 2018; Wang et al, 2018a; Eng et al, 2019; Saka et al, 2019; Nguyen et al, 2020; Su et al, 2020; Alon et al, 2021)). For methods that rely on DNA barcodes, advances in DNA barcoding protocols (both for reagents such as antibodies and cellular molecules) and barcoded array generation methods that can support even higher densities and smaller spots at lower costs will be important to improve the resolution and sensitivity (Cho et al, 2021; Chen et al, 2022b).
By now, DNA has become a go‐to barcoding molecule that helped bridge imaging and sequencing‐based approaches for spatial genomics, epigenomics, transcriptomics, and proteomics (Schueder et al, 2020). This success has been largely enabled by cost‐effective and reliable oligo synthesis (Hughes & Ellington, 2017) and the accumulated knowledge in DNA nanotechnology and computational tools and databases that improved our ability to in silico design nucleic acid probes with predictable kinetics (Zadeh et al, 2011; Beliveau et al, 2018; Mengwei et al, 2020; Passaro et al, 2020). It is foreseeable that more cost‐effective options for sequencing and DNA synthesis will further drive the progress and wider adoption of spatial omics in the future.
A large subset of spatial omics methods are not directly applicable for imaging larger volumes. Hence, performing spatial omics in 3D often requires serial sectioning with consecutive sections analyzed separately (Palmer & Alexandrov, 2015). Considering that the thickness of such sections can exceed the cell size, especially when performed on frozen tissue, this leads to discrepancies between sections that cannot be compensated. Methods that enable 3D spatial omics in thick samples (organoids, tissues, whole organs, and organisms), including tissue clearing and embedding approaches, reagent delivery and fluidics, new fast optical systems and image processing methods, hold great potential to be integrated with spatial omics. Some examples of interesting approaches in this direction include: Chung & Deisseroth (2013), Murray et al (2015), Pan et al (2016), Ku et al (2016), Wang et al (2018b), Park et al (2018), preprint: Choi et al (2019), Perens et al (2020) and Bhatia et al (2022).
The introduction of high‐resolution mass spectrometry (HRMS) was a key enabler for spatial metabolomics, lipidomics, and glycomics, which predominantly use imaging mass spectrometry (Palmer et al, 2016). The high spectral resolution enabled metabolite assignment with a substantially higher confidence even without the need for MS/MS fragmentation that is often impractical in spatial applications. Over the past years, all major imaging mass spectrometry vendors have introduced HRMS‐based instrumentation for spatial metabolomics analyses. We expect the use of imaging HRMS to continue supporting a rapid evolution of spatial metabolomics, lipidomics, and fluxomics. Nevertheless, the field still faces challenges including sensitivity, unwanted metabolite degradation and fragmentation, metabolite identification, and data interpretation that will likely drive future experimental and computational developments.
Reproducibility and commercialization
Within the rapidly growing field of spatial omics with multiple technologies still being in the early phases of development, a key practical challenge is to increase the reproducibility and fidelity of the results. Minor differences in sample handling, probe preparations, staining protocols, and instrumentation can introduce unwanted variations in the results, particularly when it comes to coverage, background, and sensitivity. Standardizing protocols and performing comparative analyses is complicated, due to, for example, the wide range of possible parameters for protocol optimization (including sample fixation/preservation, permeabilization, oligo probe sequences, choice of MALDI mass spectrometry matrices, and ion polarity mode) and the unique alterations that may be necessary to get the best results for specific tissues or custom instrumental setups. The importance of such optimizations is revealed by the typically iterative method development process, with newer and significantly improved versions being developed as a result of seemingly small adjustments to the protocols (e.g., slide‐seq v1 vs. v2, SeqFISH vs. SeqFISH+, and MALDI vs. MALDI‐2). Compared with more mature omics approaches, spatial omics can be still considered at its infancy, meaning that most methods and protocols are at a relatively nascent state and are expected to be substantially improved as they become more widely used. Increasing access to automation, streamlining of advanced multistep technological workflows and a growing popularity of microfluidics, barcoding technologies, and imaging mass spectrometry have been improving the scalability and reproducibility of the methods and is expected to drive the field forward. Reliable and compact automation systems that can help screening of sample preparation conditions with custom protocols, while supporting different sample formats and delivering high reproducibility constitutes a high unmet need.
Commercialization of methods developed in academic laboratories has been a major driver of high‐pace method popularization and helped lower the entry barrier for new users who would like to use these methods for their research questions. In the last decade, we have witnessed a rapid adoption of methods where commercial end‐to‐end automation (e.g., Akoya CODEX platform, Nanostring GeoMx and COSMx systems, 10X Genomics Visium and Xenium, Resolve Biosciences Molecular Cartography, Fluidigm Hyperion Imaging System, IONpath MIBIScope, VIZGEN MERScope, Militenyi MACSima, Lunaphore COMET), optimized protocols and reagent kits (e.g., RNAscope), integrated microscopy and mass spectrometry instrumentation (e.g., Shimadzu iMScope) and data processing tools were made available. Importantly, this could not be have been achieved without academic feedback fueling constant improvement of the products and methods. Many new startups are currently entering the market with reagent kits, automated hardware solutions, software tools, AI/ML platforms, and service models that will create numerous future possibilities in establishing novel assays, data acquisition, and analysis. In a market that is getting increasingly competitive but still at an early stage in terms of scientific capabilities, it will be important to not lose this diversity of technologies (which, most often than not, offer complementary information) due to IP conflicts and lawsuits will be important to keep the field moving forward with the same speed and creative freedom and allow careful vetting and maturation of technologies and services by the scientific community.
From the academic standpoint, there would be some other critical concerns related to relying only on industrial development. From one hand, using end‐to‐end approaches and commercial reagents does in principle improve reproducibility and facilitates access to novel technologies. However, the need for using proprietary reagents, algorithms, workflows, formats, and closed‐box systems might in turn create barriers. Foremost, proprietary systems and reagents create a paywall that may cut off the wider scientific community from reproducing the data or utilizing it. Second, this barrier impedes noncommercial development, improvement and combination of the spatial omics methods. For example, lacking the knowledge of original barcodes or probe sequences in commercial kits may prevent scientists from creating new workflows that incorporate complementary methods for multi‐omics analysis. Similarly, the use of proprietary data formats prevents interoperability and locks users in the limited software ecosystem offered by a vendor, thus inhibiting uptake in the long term. Moreover, the overlapping development time line of similar methods in both academic and commercial setups may create a version confusion in which emerging data may be attributed to previously published versions of the methods and protocols. These may be different from the commercial workflow and expectedly modified during conversion of early academic intellectual property to marketable products, making it hard to trace data‐protocol links. This may constrain the systematic accumulation of the new knowledge and create reproducibility issues. Finally, the risk of monopoly creation and price inflation may contribute to increasing the already high prices of spatial omics methods and thus hamper their accessibility. In summary, although commercialization can drive broader adoption, increased access, utility, and reproducibility of these methods, it is important for the field to create and value a rich ecosystem of technologies and vendors, and to support the open and free exchange of protocols and software whenever possible.
Going forward, and to ensure a sustainable growth of the field, it will be critical to empower open science while fostering academic‐industry collaborations. This can help address the major practical challenges, including the cost and throughput of the approaches significantly limiting the scalability, and thus ensure wide‐spread access and translation of the spatial omics approaches.
Data handling and computation
The development and application of high‐accuracy image segmentation methods as well as the adaptation of analysis tools originally utilized in single‐cell omics for spatial omics data have been instrumental during the early stages of the field. However, spatial omics data pose distinct computational challenges (Hériché et al, 2019; Alexandrov, 2020; Lähnemann et al, 2020) due to the added dimensions and increased data size, and the special nature of the data, which is often different to the bulk omics with respect to coverage, sensitivity, level of noise, and overall amount of represented information. Single‐cell‐focused tools also offer limited utility for leveraging new opportunities provided by the spatial content of the data.
The large size of spatial datasets imposes heavy requirements on data handling, infrastructure, and computational performance. Moreover, the unique aspects of spatial data including the spatial information, need for custom visualization, and often custom data formats require novel data frameworks and repositories focused on spatial omics data. Previously generated datasets and atlases such as Human Protein Atlas (Uhlén et al, 2015; Thul et al, 2017) or Allen Brain Atlases (Ortiz et al, 2020; Viana et al, 2023) have become very useful as references and frameworks for the spatial omics data to build on. Creating new repositories that can support deposition of both the raw and processed data will not only provide reference platforms but also enable benchmarking and data reprocessing and analysis with future computational tools. Ideally such spatial omics repositories shall embed advanced annotation, and analysis tools to collectively provide broader insights, as demonstrated by the METASPACE platform for spatial metabolomics (preprint: Alexandrov et al, 2019). For this a future‐proof implementation of unified nonproprietary data formats, annotations and metadata structures will be crucial. Importantly, increasing data size and complexity will likely require next‐generation data formats (Moore et al, 2023) and approaches for data management and analysis (such as preprint: Marconato et al, 2023; Walter et al, 2023).
Another challenge is the integration of multiple datasets from the same spatial omics technologies. The integration of sequencing‐based data into meta‐studies is a common practice. The integration of single‐cell transcriptomics data has been successfully demonstrated (Luecken et al, 2022; Argelaguet et al, 2021), as has been the combination of single‐cell atlases and spatially resolved datasets (Lohoff et al, 2022). However, the integration has been challenging for spatial omics datasets. The integration of image‐based data requires appropriate methods of registration and normalization, and the continuous nature of imaging data has to be taken into account. Another critical component is to have anchors that can link datasets, such as mapping one image to the other to be able to perform meta‐analyses. However, there are not yet established anchor points that can be used for normalizing, analyzing and comparing samples, limiting the utility of the data that are available in databases. The challenge is exacerbated if different data modalities are to be integrated—even if one measures the protein and transcript levels for the same genes, given the limited protein‐transcript correlation. We anticipate that addressing this challenge will be an active area of method development in the next few years. In this context, it will be essential to have adequate data and metadata standards (Schapiro et al, 2022b) as well as benchmarks (Lähnemann et al, 2020) to rigorously assess strengths and weaknesses of each method. As a means to leverage such benchmarks to accelerate the development of methods in an unbiased way, crowdsourced competitions (Tanevski et al, 2020) should be considered, complemented by a broader acceptance in funding benchmarking efforts.
Spatial omics research taps into a broad range of disciplines, which requires a higher level of interdisciplinarity and broader expertise. This creates a need for coordinated large‐scale efforts not only to generate and interpret the data but also to improve the technologies and make both the methods and the data more accessible and reproducible. In this regard, the attention of public and private funding bodies such as European Commission, National Institutes of Health, Chan Zuckerberg Initiative or Knut and Alice Wallenberg Foundation to create large consortia for developing and applying spatial omics approaches is critical. These consortia, including the Human Cell Atlas (HCA; Aviv et al, 2017), Human Biomolecular Atlas Program (HuBMAP; Aviv et al, 2017; HuBMAP Consortium, 2019), HTAN (Rozenblatt‐Rosen et al, 2020), KPMP (de Boer et al, 2021), LungMAP (Ardini‐Poleske et al, 2017), 4D nucleome (Dekker et al, 2017), and LifeTime (Rajewsky et al, 2020), have been instrumental in creating frameworks, standards, data repositories, analysis, and visualization tools as well as building a community of developers and users of spatial omics methods. Going forward, expanding such coordinated efforts and spreading them globally will tremendously improve the utility and accessibility of these methods. Such efforts will be critical to create the reference datasets and the infrastructure necessary to push spatial omics to the next level.
The future evolution and impact of spatial omics
The spatial omics field is currently a melting pot in which contributions from various disciplines are explored, evaluated, integrated, and implemented, to create methods that offer higher resolution and sensitivity, and multiple omics layers and sources of molecular knowledge, while being cost‐efficient and robust. The future and promise of spatial omics are indisputably bright and bold. Here, we look beyond this field and hypothesize how spatial omics will enable other areas, from a high‐level technological perspective.
Further technology development
Spatial omics is currently pushing technology development in other fields and, assuming its increased adoption and popularity, it is expected to have an even bigger and broader impact in related technological fields. It has already created an increased demand in barcoding approaches, microfluidics, and sequencing. Sequencing‐based approaches are often prohibitively expensive when applied to every pixel or spatial location, which will likely create further pressure to develop more cost‐effective sequencing methods. Furthermore, in single‐cell omics experiments, the choice of the specimen for single‐cell interrogation is often limited by the sampling method or specimen's anatomy. This bias does not necessarily provide a representative sample capturing the required heterogeneity, cell types, cell states, and phenotypes of interest. Here, spatial omics, once made accessible, can be a useful as a common preceding approach (much like flow cytometry enrichment of cell of interest before scRNA‐Seq) to provide guidance for more representative and informative selection of the samples (i.e., regions and cells of interest) for omics‐level analysis with NGS and mass spectrometry and in particular can enable interrogation of rare cell types and states. Similar to biomarker‐based presorting of the cells of interest for deeper sequencing, spatial omics could also be applied selectively only to regions/cells of interest to reduce the experimental costs and to enable classifying the cells at much higher depth, as shown by recent for spatially directed omics applications (Nichterwitz et al, 2016; Buczak et al, 2020; Hu et al, 2020; Merritt et al, 2020; preprint: Maliga et al, 2021; Kishi et al, 2022; Mund et al, 2022). Here, the development of novel barcoding technologies, integrated physical, or contextual spatial dissection capabilities, as well as microscopy‐driven mass spectrometry approaches can make performing such selection attainable at the single‐cell level and beyond.
Mass spectrometry
In mass spectrometry, the rise of spatial omics greatly stimulated the development of desorption approaches (MALDI, SIMS and DESI) that previously had a much narrower scope of applications. We will likely see more imaging mass spectrometry developments specifically focused on spatial omics applications to address the demand for higher mass resolution, sensitivity, and speed. Among key developments in this field are the transmission‐based MALDI desorption allowing for subcellular resolution (Niehaus et al, 2019), the increased use of fast analyzers such as QTOF, introduced ion mobility separation (IMS) for higher specificity and enhanced molecular identification, and modifications of ultrahigh‐resolution SIMS imaging mass spectrometry aiming to reduce unwanted fragmentation and allowing for detection of biomolecules (Pareek et al, 2020; Ganesh et al, 2021). Finally, the increase of sensitivity stimulated by the emerging single‐cell mass spectrometry capabilities by improving sample preparation, chromatography, mass spectrometry, and computational approaches will likely have a positive impact on spatial omics for ex situ but also for in situ approaches.
Sequencing‐based
Future adaptations of 3D chromatin conformation capture methods (3C, 4C, 5C, Hi‐C, Micro‐C, SPRITE and many more; Grob & Cavalli, 2018) that would go beyond the short and long‐range interactions and reveal the absolute or relative 3D spatial location of genetic sequences inside the nucleus down to single‐cell level would complement the multiplexed and combinatorial imaging‐based spatial genomics approaches (Payne et al, 2020; preprint: Takei et al, 2023) and greatly enhance our understanding of nuclear organization and gene regulation. Although still at its infancy, DNA microscopy and proximity detection approaches are also poised to create new capabilities in converting nanoscale spatial information into sequencable readouts (Schaus et al, 2017; Hoffecker et al, 2019; Weinstein et al, 2019; preprint: Gopalkrishnan et al, 2020).
Spatial multi‐omics
Beyond combining spatial location of cells and their neighborhoods with the cell types and states as typically defined by transcriptomics approaches, spatial omics is poised to integrate many more layers of information. As opposed to single‐cell approaches that operate on dissociated cells that are typically lysed/desorbed/consumed in the process, spatial omics approaches can more easily support spatial multi‐omics by applying different modalities on the same cells (Vickovic et al, 2022; Liu et al, 2023; preprint: Takei et al, 2023; Vandereyken et al, 2023; Zhang et al, 2023). For many of the existing approaches, it is a matter of optimization of the sample preparation conditions to copreserve the molecular composition and detection access for multiple components (RNA, protein, and DNA) simultaneously and more combinations will no doubt be increasingly utilized for high‐throughput experiments (Baysoy et al, 2023).
Mass spectrometry also provides multiple capabilities. In general, small molecules, lipids, glycans, and small peptides can be detected by employing broad mass range mass spectrometry, although this comes at the disadvantage of reduced sensitivity. Future combinations of multiplexed imaging‐based methods with mass spectrometry detection will create new capabilities for multimodal analysis. For example, targeted detection of transcripts using RNA‐FISH on the same tissue section after imaging mass spectrometry analyses helped resolve host–microbe interactions (Geier et al, 2020). Such applications open an avenue to combine label‐free spatial metabolomics or proteomics with other spatial omics approaches with more reports emerging (preprint: Vicari et al, 2023). In parallel, spatial multi‐omics for proteins and small molecules or lipids was demonstrated by a MALDI‐IHC approach for targeted detection of proteins by means of photocleavable mass‐tags conjugated to antibody probes, and another round of imaging mass spectrometry for high‐plex detection of peptide tag in situ (Lim et al, 2023).
A key future area of is subcellular spatial multi‐omics (Park et al, 2022), where integration of morphological cell features from microscopy, highly‐multiplexed subcellular detection of gene and protein expression, and metabolite and lipid localizations would be needed. While microscopy‐based approaches for spatial profiling of gene and protein expression are typically the most straightforward for achieving higher resolution without sacrificing sensitivity, further complementation with emerging subcellular technologies for spatial barcoding and subcellular MALDI‐imaging approaches in spatial metabolomics, lipidomics, and soon glycomics can provide interesting new dimensions.
Artificial intelligence and machine learning
Currently, spatial omics benefits from the rapidly growing computational developments in particular in Artificial Intelligence and Machine Learning (AI/ML), including via bespoke methods, as outlined above. It is easy to imagine a situation in the future in which, in return, spatial omics will enable novel developments in computational biology. Indeed, predicting complex biological systems needs big, diverse, and representative data properly annotated with a desired class, state, or phenotype especially when using machine learning. Here, spatial omics can be a game changer by providing more data than what is possible to collect by bulk omics and with the native context information, and potentially in an easier, cheaper, or faster manner from a large number of cells as compared to the matching single‐cell omics. Taking into account the ongoing revolution of generative machine learning models (Lopez et al, 2020; preprint: Cui et al, 2023), spatial omics combining location and molecular profiles can serve as a perfect source of large data to train future generative machine learning models predicting cell images, phenotypes, and states.
Modeling
Besides providing data for future AI/ML, we expect spatial omics to become a major enabler for dynamic mechanistic modeling. Bottom‐up, mechanistic modeling of intracellular processes already benefits from the availability of bulk and, as of recently, single‐cell data (Garrido‐Rodriguez et al, 2022; Hrovatin et al, 2022). At the same time, top‐down models describing the physiology of organisms at a macroscopic level typically lack molecular characterization with spatial resolution. Here, spatial omics have the potential to provide the missing molecular data to bridge top‐down and bottom‐up modeling approaches, providing valuable information about subcellular compartmentalization, cell–cell interactions, and molecular factors controlling these interactions, molecular tissue architecture, and chemical microenvironment. This will enable building models that span across biological scales, and integrate intracellular processes and physiological responses. These models could then be used to simulate the effect of molecular interventions, such as a mutation or a drug treatment. The large size of this data and the need to account for spatial dimensions in modeling will likely require using high‐performance computing and thus, availability of the next‐generation computing resources for life scientists. These models will probably be first applicable to simpler systems (organoids, organs‐on‐a‐chip, 3D printed organs, and xenografts) that can be used as proxies of complex organs. Expanding spatial omics into 3D to support these complex models will provide new ways to characterize them better and improve their accuracy. It will also offer the possibility to perform extensive manipulation of the system under controlled conditions, potentially transforming drug screening and personalized therapy development. Thereby, spatial omics will be critical to develop a virtual cell representing a real biological cell in its full complexity and heterogeneity and encompassing possible cellular and molecular programs is a long‐demanded yet ambitious future aim, and on the long term, virtual tissues, virtual organs, virtual organisms (including digital twins in medicine), and virtual ecosystems. Spatial omics datasets and atlases that are being generated, in particular from major systematic efforts, can provide information for creating such virtual biological systems as well as for validating their ability to mimic real systems. We further expect that efforts such as Allen Cell Explorer (Viana et al, 2023) and Human Protein Atlas (Uhlén et al 2015) would be important contributions to populate virtual cells with subcellular organizational details and to explore this information in 3D.
Clinical and beyond
From a clinical perspective, spatial omics provides molecular data that can revolutionize histology and pathology, which currently exploits conventional stainings (e.g., hematoxylin and eosin; H&E) or individual markers stained with immunohistochemistry followed by microscopy and statistical and machine learning. This is particularly demanding since the conventional staining and microscopy approaches can lack resolution in the disease stages or types. On the contrary, multiple large‐scale characterization studies by spatial omics revealed the depths of spatial and cell heterogeneity in various indications including cancer (Lewis et al, 2021) and human myocardial infarction (Kuppe et al, 2022). Moreover, spatial omics already helped pinpointing specific tissue architecture, cell composition, and functional states of cells associated with the therapy response (Zhao et al, 2019; Helmink et al, 2020). Thus, using rich molecular information far exceeding that provided by cytochemistry and histochemistry staining can enable an improved classification and stratification of patients and eventually lead to an improvement of treatments and enable precision medicine. Although it is still early days for such approaches, the first projects following this strategy have already provided promising preliminary results (Irmisch et al, 2021). At the same time, machine learning models can predict spatially resolved single‐cell profiles from H&E images, strengthening the link between the phenotype and omics profiles (preprint: Comiter et al, 2023). However, it remains to be seen which cellular programs, or intensities of genes, proteins, or metabolites can be predicted and how this information can be used in applications. Furthermore, spatial transcriptomics and histology can be merged using deep learning to combine the molecular coverage of the former with the spatial resolution of the later (Bergenstråhle et al, 2022). Overall, spatial omics carry promise for addressing clinical questions already in these early days while the technologies are still maturing (Liu et al, 2022; Zhang et al, 2022). The search is open for finding the best‐fitting applications and for improving technologies with respect to their scalability, cost, and robustness.
Spatial omics methods are also poised to reveal novel insights into the role and regulation of the microbiome and the human ecosystems by allowing in situ omics investigation of hosts and microbes and microbial communities (Tropini et al, 2017; preprint: Lötstedt et al, 2022; preprint: Saarenpää et al, 2022). Similarly, toxicology, infection biology, and microbial pathogenesis fields would benefit greatly from spatial omics approaches, especially at a high resolution (Rendeiro et al, 2021; Lempke et al, 2023). Although the initial applications were largely focused on tissue samples from mouse models and human donors en route to clinical implementations, we expect that spatial omics methods will be increasingly used for environmental samples and nonmodel organisms, and offer new insights for planetary biology and ecosystems (Liang et al, 2018; Cao et al, 2023).
Conclusions
With this multifold promise of spatial omics addressing the growing demand for investigating biology in its spatial context, and fueled by the recent technological progress and breakthroughs, the rapid progress of technological developments in this cutting‐edge field is gathering steam. Similar to the ongoing merging of imaging‐focused and sequencing‐focused efforts in spatial transcriptomics, one can imagine spatial omics getting more closely integrated into the broader field of omics. A further convergence will likely be happening between spatial and single‐cell omics with the continuing increase of the spatial resolution across all spatial omics. This can lead to a scenario where the instrumentation and methodological differences between spatial vs. single‐cell vs. bulk omics are blurred or even nonexisting. This would create better opportunities for investigating biology and addressing medical challenges with future “omics” encompassing spatial, single‐cell, and subcellular capacities where even using the term “spatial omics” would sound as strange as “spatial microscopy.”
Author contributions
Theodore Alexandrov: Conceptualization; writing – original draft; writing – review and editing. Julio Saez‐Rodriguez: Conceptualization; writing – original draft; writing – review and editing. Sinem Saka: Conceptualization; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
JS‐R has received funding from GSK, Pfizer and Sanofi and fees/honoraria from Travere Therapeutics, Pfizer, Grunenthal, Stadapharm and Astex. SKS is an inventor on patent applications related to some of the DNA barcoding and imaging methods described here, is a consulting scientific co‐founder and shareholder for Digital Biology, Inc., and receives research funding from Cellzome, a GSK company. TA is an inventor of several patents in the field of imaging mass spectrometry and leads a startup‐in‐incubation in the area of single‐cell metabolomics at the BioInnovation Institute.
Acknowledgments
This work was supported by core funding from European Molecular Biology Laboratory (SKS and TA). TA acknowledges funding from the European Research Council (Consolidator grant #773098, Proof of Concept grant no. 101101077), Michael J Fox Foundation, and Swiss National Foundation (Sinergia grant PROMETEX). We thank Martijn Molenaar, Kevin Titeca (EMBL), and Denis Schapiro (Heidelberg University) for providing their comments on the manuscript draft.
Mol Syst Biol. (2023) 19: e10571
Contributor Information
Theodore Alexandrov, Email: theodore.alexandrov@embl.de.
Julio Saez‐Rodriguez, Email: pub.saez@uni-heidelberg.de.
Sinem K Saka, Email: sinem.saka@embl.de.
References
- Achim K, Pettit J‐B, Saraiva LR, Gavriouchkina D, Larsson T, Arendt D, Marioni JC (2015) High‐throughput spatial mapping of single‐cell RNA‐seq data to tissue of origin. Nat Biotechnol 33: 503–509 [DOI] [PubMed] [Google Scholar]
- Agasti SS, Wang Y, Schueder F, Sukumar A, Jungmann R, Yin P (2017) DNA‐barcoded labeling probes for highly multiplexed Exchange‐PAINT imaging. Chem Sci 8: 3080–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov T (2020) Spatial metabolomics and imaging mass spectrometry in the age of artificial intelligence. Annu Rev Biomed Data Sci 3: 61–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov T, Ovchnnikova K, Palmer A, Kovalev V (2019) METASPACE: a community‐populated knowledge base of spatial metabolomes in health and disease. bioRxiv 10.1101/539478 [PREPRINT] [DOI]
- Alon S, Goodwin DR, Sinha A, Wassie AT, Chen F, Daugharthy ER, Bando Y, Kajita A, Xue AG, Marrett K et al (2021) Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science 371: eaax2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Bergenstråhle J, Asp M, Bergenstråhle L, Jurek A, Fernández Navarro J, Lundeberg J (2020) Single‐cell and spatial transcriptomics enables probabilistic inference of cell type topography. Commun Biol 3: 565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S et al (2014) Multiplexed ion beam imaging of human breast tumors. Nat Med 20: 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardini‐Poleske ME, Clark RF, Ansong C, Carson JP, Corley RA, Deutsch GH, Hagood JS, Kaminski N, Mariani TJ, Potter SS et al (2017) LungMAP: the Molecular Atlas of Lung Development Program. Am J Physiol Lung Cell Mol Physiol 313: L733–L740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argelaguet R, Cuomo ASE, Stegle O, Marioni JC (2021) Computational principles and challenges in single‐cell data integration. Nat Biotechnol 39: 1202–1215 [DOI] [PubMed] [Google Scholar]
- Armingol E, Officer A, Harismendy O, Lewis NE (2021) Deciphering cell‐cell interactions and communication from gene expression. Nat Rev Genet 22: 71–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnol D, Schapiro D, Bodenmiller B, Saez‐Rodriguez J, Stegle O (2019) Modeling cell‐cell interactions from spatial molecular data with spatial variance component analysis. Cell Rep 29: 202–211.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv R, Teichmann SA, Lander ES, Ido A, Christophe B (2017) The Human Cell Atlas. Elife 6: e27041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bageritz J, Willnow P, Valentini E, Leible S, Boutros M, Teleman AA (2019) Gene expression atlas of a developing tissue by single cell expression correlation analysis. Nat Methods 16: 750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DA, Dileep V, Handa T, Ohkawa Y, Kimura H, Henikoff S, Gilbert DM (2021) High‐throughput single‐cell epigenomic profiling by targeted insertion of promoters (TIP‐seq). J Cell Biol 220: e202103078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysoy A, Bai Z, Satija R, Fan R (2023) The technological landscape and applications of single‐cell multi‐omics. Nat Rev Mol Cell Biol 1–19 10.1038/s41580-023-00615-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau BJ, Joyce EF, Apostolopoulos N, Yilmaz F, Fonseka CY, McCole RB, Chang Y, Li JB, Senaratne TN, Williams BR et al (2012) Versatile design and synthesis platform for visualizing genomes with Oligopaint FISH probes. Proc Natl Acad Sci USA 109: 21301–21306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau BJ, Boettiger AN, Avendaño MS, Jungmann R, McCole RB, Joyce EF, Kim‐Kiselak C, Bantignies F, Fonseka CY, Erceg J et al (2015) Single‐molecule super‐resolution imaging of chromosomes and in situ haplotype visualization using Oligopaint FISH probes. Nat Commun 6: 7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau BJ, Kishi JY, Nir G, Sasaki HM, Saka SK, Nguyen SC, Wu C‐T, Yin P (2018) OligoMiner provides a rapid, flexible environment for the design of genome‐scale oligonucleotide in situ hybridization probes. Proc Natl Acad Sci USA 115: E2183–E2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Chetrit N, Niu X, Swett AD, Sotelo J, Jiao MS, Stewart CM, Potenski C, Mielinis P, Roelli P, Stoeckius M et al (2023) Integration of whole transcriptome spatial profiling with protein markers. Nat Biotechnol 41: 788–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenstråhle L, He B, Bergenstråhle J, Abalo X, Mirzazadeh R, Thrane K, Ji AL, Andersson A, Larsson L, Stakenborg N et al (2022) Super‐resolved spatial transcriptomics by deep data fusion. Nat Biotechnol 40: 476–479 [DOI] [PubMed] [Google Scholar]
- Bhatia HS, Brunner A‐D, Öztürk F, Kapoor S, Rong Z, Mai H, Thielert M, Ali M, Al‐Maskari R, Paetzold JC et al (2022) Spatial proteomics in three‐dimensional intact specimens. Cell 185: 5040–5058.e19 [DOI] [PubMed] [Google Scholar]
- Biancalani T, Scalia G, Buffoni L, Avasthi R, Lu Z, Sanger A, Tokcan N, Vanderburg CR, Segerstolpe A, Zhang M et al (2020) Deep learning and alignment of spatially‐resolved whole transcriptomes of single cells in the mouse brain with Tangram. bioRxiv 10.1101/2020.08.29.272831 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Biancalani T, Scalia G, Buffoni L, Avasthi R, Lu Z, Sanger A, Tokcan N, Vanderburg CR, Segerstolpe Å, Zhang M et al (2021) Deep learning and alignment of spatially resolved single‐cell transcriptomes with Tangram. Nat Methods 18: 1352–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer IH, Alpers CE, Azeloglu EU, Balis UGJ, Barasch JM, Barisoni L, Blank KN, Bomback AS, Brown K, Dagher PC et al (2021) Rationale and design of the Kidney Precision Medicine Project. Kidney Int 99: 498–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borm LE, Mossi Albiach A, Mannens CCA, Janusauskas J, Özgün C, Fernández‐García D, Hodge R, Castillo F, Hedin CRH, Villablanca EJ et al (2023) Scalable in situ single‐cell profiling by electrophoretic capture of mRNA using EEL FISH. Nat Biotechnol 41: 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, Gonzalez A, Berg‐Lyon D, Ackermann G, Moeller Christensen GJ, Nakatsuji T et al (2015) Molecular cartography of the human skin surface in 3D. Proc Natl Acad Sci USA 112: E2120–E2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AP, Heeren RMA, Ellis SR (2019) Advances in mass spectrometry imaging enabling observation of localised lipid biochemistry within tissues. Trends Analyt Chem 120: 115197 [Google Scholar]
- Buczak K, Kirkpatrick JM, Truckenmueller F, Santinha D, Ferreira L, Roessler S, Singer S, Beck M, Ori A (2020) Spatially resolved analysis of FFPE tissue proteomes by quantitative mass spectrometry. Nat Protoc 15: 2956–2979 [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ (2013) Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA‐binding proteins and nucleosome position. Nat Methods 10: 1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable DM, Murray E, Zou LS, Goeva A, Macosko EZ, Chen F, Irizarry RA (2021) Robust decomposition of cell type mixtures in spatial transcriptomics. Nat Biotechnol 40: 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang Z, Nie Q (2020) Inferring spatial and signaling relationships between cells from single cell transcriptomic data. Nat Commun 11: 2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang Z, Zhao Y, Almet AA, Stabell A, Ramos R, Plikus MV, Atwood SX, Nie Q (2023) Screening cell–cell communication in spatial transcriptomics via collective optimal transport. Nat Methods 20: 218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Zuo W, Wang L, Chen J, Qu Z, Jin F, Dai L (2023) Spatial profiling of microbial communities by sequential FISH with error‐robust encoding. Nat Commun 14: 1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X (2015) RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348: aaa6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM (2016a) Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166: 1324–1337.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shen Y, Draper W, Buenrostro JD, Litzenburger U, Cho SW, Satpathy AT, Carter AC, Ghosh RP, East‐Seletsky A et al (2016b) ATAC‐see reveals the accessible genome by transposase‐mediated imaging and sequencing. Nat Methods 13: 1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Guillaume‐Gentil O, Rainer PY, Gäbelein CG, Saelens W, Gardeux V, Klaeger A, Dainese R, Zachara M, Zambelli T et al (2022a) Live‐seq enables temporal transcriptomic recording of single cells. Nature 608: 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, Qiu X, Yang J, Xu J, Hao S et al (2022b) Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball‐patterned arrays. Cell 185: 1777–1792.e21 [DOI] [PubMed] [Google Scholar]
- Cho C‐S, Xi J, Si Y, Park S‐R, Hsu J‐E, Kim M, Jun G, Kang HM, Lee JH (2021) Microscopic examination of spatial transcriptome using Seq‐Scope. Cell 184: 3559–3572.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HMT, Chang JY, Trinh LA, Padilla JE, Fraser SE, Pierce NA (2010) Programmable in situ amplification for multiplexed imaging of mRNA expression. Nat Biotechnol 28: 1208–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HMT, Beck VA, Pierce NA (2014) Next‐generation in situ hybridization chain reaction: higher gain, lower cost, greater durability. ACS Nano 8: 4284–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HMT, Schwarzkopf M, Fornace ME, Acharya A, Artavanis G, Stegmaier J, Cunha A, Pierce NA (2018) Third‐generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145: dev165753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Jung HY, Ruelas L, Feng G, Chung K (2019) Ultrafast immunostaining of organ‐scale tissues for scalable proteomic phenotyping. bioRxiv 10.1101/660373 [PREPRINT] [DOI]
- Chung K, Deisseroth K (2013) CLARITY for mapping the nervous system. Nat Methods 10: 508–513 [DOI] [PubMed] [Google Scholar]
- Comiter C, Vaishnav ED, Ciampricotti M, Li B, Yang Y, Rodig SJ, Turner M, Pfaff KL, Jané‐Valbuena J, Slyper M et al (2023) Inference of single cell profiles from histology stains with the Single‐Cell omics from Histology Analysis Framework (SCHAF). bioRxiv 10.1101/2023.03.21.533680 [PREPRINT] [DOI]
- Cui H, Wang C, Maan H, Pang K, Luo F, Wang B (2023) sc GPT: towards building a foundation model for single‐cell multi‐omics using generative AI. bioRxiv 10.1101/2023.04.30.538439 [PREPRINT] [DOI] [PubMed]
- Dardani I, Emert BL, Goyal Y, Jiang CL, Kaur A, Lee J, Rouhanifard SH, Alicea GM, Fane ME, Xiao M et al (2022) ClampFISH 2.0 enables rapid, scalable amplified RNA detection in situ . Nat Methods 19: 1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Belmont AS, Guttman M, Leshyk VO, Lis JT, Lomvardas S, Mirny LA, O'Shea CC, Park PJ, Ren B et al (2017) The 4D nucleome project. Nature 549: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Bartosovic M, Ma S, Zhang D, Kukanja P, Xiao Y, Su G, Liu Y, Qin X, Rosoklija GB et al (2022) Spatial profiling of chromatin accessibility in mouse and human tissues. Nature 609: 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks RM, Pierce NA (2004) Triggered amplification by hybridization chain reaction. Proc Natl Acad Sci USA 101: 15275–15278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries R, Zhu Q, Dong R, Eng C‐HL, Li H, Liu K, Fu Y, Zhao T, Sarkar A, Bao F et al (2021) Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol 22: 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Su Y, Qian C, Yuan D, Miao K, Lee D, Ng AHC, Wijker RS, Ribas A, Levine RD et al (2020) Raman‐guided subcellular pharmaco‐metabolomics for metastatic melanoma cells. Nat Commun 11: 4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley TPJ, Watson R, Griffin JL, Dupree P, Lilley KS (2004) Localization of organelle proteins by isotope tagging (LOPIT). Mol Cell Proteomics 3: 1128–1134 [DOI] [PubMed] [Google Scholar]
- Edsgärd D, Johnsson P, Sandberg R (2018) Identification of spatial expression trends in single‐cell gene expression data. Nat Methods 15: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosua‐Bayes M, Nieto P, Mereu E, Gut I, Heyn H (2021) SPOTlight: seeded NMF regression to deconvolute spatial transcriptomics spots with single‐cell transcriptomes. Nucleic Acids Res 49: e50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C‐HL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, Yun J, Cronin C, Karp C, Yuan G‐C et al (2019) Transcriptome‐scale super‐resolved imaging in tissues by RNA seqFISH. Nature 568: 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femino AM, Fay FS, Fogarty K, Singer RH (1998) Visualization of single RNA transcripts in situ . Science 280: 585–590 [DOI] [PubMed] [Google Scholar]
- Fischer DS, Schaar AC, Theis FJ (2023) Modeling intercellular communication in tissues using spatial graphs of cells. Nat Biotechnol 41: 332–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Sun L, Dong R, Chen JY, Silakit R, Condon LF, Lin Y, Lin S, Palmiter RD, Gu L (2022) Polony gels enable amplifiable DNA stamping and spatial transcriptomics of chronic pain. Cell 185: 4621–4633.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh S, Hu T, Woods E, Allam M, Cai S, Henderson W, Coskun AF (2021) Spatially resolved 3D metabolomic profiling in tissues. Sci Adv 7: eabd0957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Alonso L, Lorenzi V, Mazzeo CI, Alves‐Lopes JP, Roberts K, Sancho‐Serra C, Engelbert J, Marečková M, Gruhn WH, Botting RA et al (2022) Single‐cell roadmap of human gonadal development. Nature 607: 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido‐Rodriguez M, Zirngibl K, Ivanova O, Lobentanzer S, Saez‐Rodriguez J (2022) Integrating knowledge and omics to decipher mechanisms via large‐scale models of signaling networks. Mol Syst Biol 18: e11036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier B, Sogin EM, Michellod D, Janda M, Kompauer M, Spengler B, Dubilier N, Liebeke M (2020) Spatial metabolomics of in situ host–microbe interactions at the micrometre scale. Nat Microbiol 5: 498–510 [DOI] [PubMed] [Google Scholar]
- Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, Can A, Corwin A, Dinn S, Filkins RJ et al (2013) Highly multiplexed single‐cell analysis of formalin‐fixed, paraffin‐embedded cancer tissue. Proc Natl Acad Sci USA 110: 11982–11987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN (2012) Histo‐cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar S, Lin Y, Su X, Lin DM, Patrick E, Han Z‐G, Marioni JC, Yang JYH (2020) Investigating higher‐order interactions in single‐cell data with scHOT. Nat Methods 17: 799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schüffler PJ, Grolimund D, Buhmann JM, Brandt S et al (2014) Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 11: 417–422 [DOI] [PubMed] [Google Scholar]
- Go CD, Knight JDR, Rajasekharan A, Rathod B, Hesketh GG, Abe KT, Youn J‐Y, Samavarchi‐Tehrani P, Zhang H, Zhu LY et al (2021) A proximity‐dependent biotinylation map of a human cell. Nature 595: 120–124 [DOI] [PubMed] [Google Scholar]
- Goltsev Y, Samusik N, Kennedy‐Darling J, Bhate S, Hale M, Vazquez G, Black S, Nolan GP (2018) Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174: 968–981.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalkrishnan N, Punthambaker S, Schaus TE, Church GM, Yin PA (2020) DNA nanoscope that identifies and precisely localizes over a hundred unique molecular features with nanometer accuracy. bioRxiv 10.1101/2020.08.27.271072 [PREPRINT] [DOI]
- Greenwald NF, Miller G, Moen E, Kong A, Kagel A (2022) Whole‐cell segmentation of tissue images with human‐level performance using large‐scale data annotation and deep learning. Nat Biotechnol 40: 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob S, Cavalli G (2018) Technical review: a Hitchhiker's guide to chromosome conformation capture. Methods Mol Biol 1675: 233–246 [DOI] [PubMed] [Google Scholar]
- Gut G, Herrmann MD, Pelkmans L (2018) Multiplexed protein maps link subcellular organization to cellular states. Science 361: eaar7042 [DOI] [PubMed] [Google Scholar]
- Gyllborg D, Langseth CM, Qian X, Choi E, Salas SM, Hilscher MM, Lein ES, Nilsson M (2020) Hybridization‐based in situ sequencing (HybISS) for spatially resolved transcriptomics in human and mouse brain tissue. Nucleic Acids Res 48: e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase C, Gustafsson K, Mei S, Yeh S‐C, Richter D, Milosevic J, Turcotte R, Kharchenko PV, Sykes DB, Scadden DT et al (2022) Image‐seq: spatially resolved single‐cell sequencing guided by in situ and in vivo imaging. Nat Methods 19: 1622–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Stuart T, Kowalski M, Choudhary S, Hoffman P, Hartman A, Srivastava A, Molla G, Madad S, Fernandez‐Granda C et al (2022) Dictionary learning for integrative, multimodal, and scalable single‐cell analysis. bioRxiv 10.1101/2022.02.24.481684 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade‐Feldman M, Blando J, Han G et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577: 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hériché J‐K, Alexander S, Ellenberg J (2019) Integrating imaging and omics: computational methods and challenges. Annu Rev Biomed Data Sci 2: 175–197 [Google Scholar]
- Heumos L, Schaar AC, Lance C, Litinetskaya A, Drost F, Zappia L, Lücken MD, Strobl DC, Henao J, Curion F et al (2023) Best practices for single‐cell analysis across modalities. Nat Rev Genet 24: 550–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey JW, Neumann EK, Radtke AJ, Camarillo JM, Beuschel RT, Albanese A, McDonough E, Hatler J, Wiblin AE, Fisher J et al (2021) Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody‐based imaging. Nat Methods 19: 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffecker IT, Yang Y, Bernardinelli G, Orponen P, Högberg B (2019) A computational framework for DNA sequencing microscopy. Proc Natl Acad Sci USA 116: 19282–19287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrovatin K, Fischer DS, Theis FJ (2022) Toward modeling metabolic state from single‐cell transcriptomics. Mol Metab 57: 101396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KH, Eichorst JP, McGinnis CS, Patterson DM, Chow ED, Kersten K, Jameson SC, Gartner ZJ, Rao AA, Krummel MF (2020) ZipSeq: barcoding for real‐time mapping of single cell transcriptomes. Nat Methods 17: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Li X, Coleman K, Schroeder A, Ma N, Irwin DJ, Lee EB, Shinohara RT, Li M (2021) SpaGCN: integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat Methods 18: 1342–1351 [DOI] [PubMed] [Google Scholar]
- HuBMAP Consortium (2019) The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Ellington AD (2017) Synthetic DNA synthesis and assembly: putting the synthetic in synthetic biology. Cold Spring Harb Perspect Biol 9: a023812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J et al (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551: 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmisch A, Bonilla X, Chevrier S, Lehmann K‐V, Singer F, Toussaint NC, Esposito C, Mena J, Milani ES, Casanova R et al (2021) The Tumor Profiler Study: integrated, multi‐omic, functional tumor profiling for clinical decision support. Cancer Cell 39: 288–293 [DOI] [PubMed] [Google Scholar]
- Jacquemet G, Carisey AF, Hamidi H, Henriques R, Leterrier C (2020) The cell biologist's guide to super‐resolution microscopy. J Cell Sci 133: jcs240713 [DOI] [PubMed] [Google Scholar]
- Jungmann R, Avendaño MS, Woehrstein JB, Dai M, Shih WM, Yin P (2014) Multiplexed 3D cellular super‐resolution imaging with DNA‐PAINT and Exchange‐PAINT. Nat Methods 11: 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wählby C, Nilsson M (2013) In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods 10: 857–860 [DOI] [PubMed] [Google Scholar]
- Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S, Yang S‐R, Kurian A, Van Valen D, West R et al (2018) A structured tumor‐immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174: 1373–1387.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstens HM, Poddighe PJ, Hanselaar AG (1995) A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem 43: 347–352 [DOI] [PubMed] [Google Scholar]
- Khatib‐Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM (2006) Direct molecular analysis of whole‐body animal tissue sections by imaging MALDI mass spectrometry. Anal Chem 78: 6448–6456 [DOI] [PubMed] [Google Scholar]
- Kishi JY, Lapan SW, Beliveau BJ, West ER, Zhu A, Sasaki HM, Saka SK, Wang Y, Cepko CL, Yin P (2019) SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat Methods 16: 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi JY, Liu N, West ER, Sheng K, Jordanides JJ, Serrata M, Cepko CL, Saka SK, Yin P (2022) Light‐Seq: light‐directed in situ barcoding of biomolecules in fixed cells and tissues for spatially indexed sequencing. Nat Methods 19: 1393–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW (2015) Droplet barcoding for single‐cell transcriptomics applied to embryonic stem cells. Cell 161: 1187–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleshchevnikov V, Shmatko A, Dann E, Aivazidis A, King HW, Li T, Lomakin A, Kedlian V, Jain MS, Park JS et al (2020) Comprehensive mapping of tissue cell architecture via integrated single cell and spatial transcriptomics. bioRxiv 10.1101/2020.11.15.378125 [PREPRINT] [DOI]
- Ku T, Swaney J, Park J‐Y, Albanese A, Murray E, Cho JH, Park Y‐G, Mangena V, Chen J, Chung K (2016) Multiplexed and scalable super‐resolution imaging of three‐dimensional protein localization in size‐adjustable tissues. Nat Biotechnol 34: 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppe C, Ramirez Flores RO, Li Z, Hayat S, Levinson RT, Liao X, Hannani MT, Tanevski J, Wünnemann F, Nagai JS et al (2022) Spatial multi‐omic map of human myocardial infarction. Nature 608: 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähnemann D, Köster J, Szczurek E, McCarthy DJ, Hicks SC, Robinson MD, Vallejos CA, Campbell KR, Beerenwinkel N, Mahfouz A et al (2020) Eleven grand challenges in single‐cell data science. Genome Biol 21: 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Frisén J, Lundeberg J (2021) Spatially resolved transcriptomics adds a new dimension to genomics. Nat Methods 18: 15–18 [DOI] [PubMed] [Google Scholar]
- Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, Terry R, Jeanty SSF, Li C, Amamoto R et al (2014) Highly multiplexed subcellular RNA sequencing in situ . Science 343: 1360–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Marco Salas S, Gyllborg D, Nilsson M (2022) Direct RNA targeted in situ sequencing for transcriptomic profiling in tissue. Sci Rep 12: 7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempke S, May D, Ewald SE (2023) Microbial pathogenesis in the era of spatial omics. Infect Immun 91: e0044222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Asselin‐Labat M‐L, Nguyen Q, Berthelet J, Tan X, Wimmer VC, Merino D, Rogers KL, Naik SH (2021) Spatial omics and multiplexed imaging to explore cancer biology. Nat Methods 18: 997–1012 [DOI] [PubMed] [Google Scholar]
- Li B, Zhang W, Guo C, Xu H, Li L, Fang M, Hu Y, Zhang X, Yao X, Tang M et al (2022) Benchmarking spatial and single‐cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat Methods 19: 662–670 [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhu Y, Dou M, Xu K, Chu RK, Chrisler WB, Zhao R, Hixson KK, Kelly RT (2018) Spatially resolved proteome profiling of <200 cells from tomato fruit pericarp by integrating laser‐capture microdissection with nanodroplet sample preparation. Anal Chem 90: 11106–11114 [DOI] [PubMed] [Google Scholar]
- Lim MJ, Yagnik G, Henkel C, Frost SF, Bien T, Rothschild KJ (2023) MALDI HiPLEX‐IHC: multiomic and multimodal imaging of targeted intact proteins in tissues. Front Chem 11: 1182404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JR, Chen YA, Campton D, Cooper J, Coy S, Yapp C, Tefft JB, McCarty E, Ligon KL, Rodig SJ et al (2023) High‐plex immunofluorescence imaging and traditional histology of the same tissue section for discovering image‐based biomarkers. Nat Cancer 4: 1036–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J‐R, Fallahi‐Sichani M, Sorger PK (2015) Highly multiplexed imaging of single cells using a high‐throughput cyclic immunofluorescence method. Nat Commun 6: 8390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J‐R, Izar B, Wang S, Yapp C, Mei S, Shah PM, Santagata S, Sorger PK (2018) Highly multiplexed immunofluorescence imaging of human tissues and tumors using t‐CyCIF and conventional optical microscopes. Elife 7: e31657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, Tebaldi T, Zhang D, Kim D, Bai Z et al (2020) High‐spatial‐resolution multi‐omics sequencing via deterministic barcoding in tissue. Cell 183: 1665–1681.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Punthambaker S, Iyer EPR, Ferrante T, Goodwin D, Fürth D, Pawlowski AC, Jindal K, Tam JM, Mifflin L et al (2021) Barcoded oligonucleotides ligated on RNA amplified for multiplexed and parallel in situ analyses. Nucleic Acids Res 49: e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang Y, Song D, Zhang L, Xu G, Hou R, Zhang Y, Chen J, Cheng Y, Liu L et al (2022) Clinical challenges of tissue preparation for spatial transcriptome. Clin Transl Med 12: e669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, DiStasio M, Su G, Asashima H, Enninful A, Qin X, Deng Y, Nam J, Gao F, Bordignon P et al (2023) High‐plex protein and whole transcriptome co‐mapping at cellular resolution with spatial CITE‐seq. Nat Biotechnol 10.1038/s41587-023-01676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi PM, Huang X, Zhu Z, Bray‐Ward P, Thomas DC, Ward DC (1998) Mutation detection and single‐molecule counting using isothermal rolling‐circle amplification. Nat Genet 19: 225–232 [DOI] [PubMed] [Google Scholar]
- Lohoff T, Ghazanfar S, Missarova A, Koulena N, Pierson N, Griffiths JA, Bardot ES, Eng C‐HL, Tyser RCV, Argelaguet R et al (2022) Integration of spatial and single‐cell transcriptomic data elucidates mouse organogenesis. Nat Biotechnol 40: 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R, Gayoso A, Yosef N (2020) Enhancing scientific discoveries in molecular biology with deep generative models. Mol Syst Biol 16: e9198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötstedt B, Stražar M, Xavier R, Regev A, Vickovic S (2022) Spatial host‐microbiome sequencing. bioRxiv 10.1101/2022.07.18.500470 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Louie KB, Bowen BP, McAlhany S, Huang Y, Price JC, Mao J‐H, Hellerstein M, Northen TR (2013) Mass spectrometry imaging for in situ kinetic histochemistry. Sci Rep 3: 1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ang CE, Zhuang X (2022) Spatially resolved epigenomic profiling of single cells in complex tissues. Cell 185: 4448–4464.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeck E, Coskun AF, Zhiyentayev T, Ahmad M, Cai L (2014) Single‐cell in situ RNA profiling by sequential hybridization. Nat Methods 11: 360–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken MD, Büttner M, Chaichoompu K, Danese A, Interlandi M, Mueller MF, Strobl DC, Zappia L, Dugas M, Colomé‐Tatché M et al (2022) Benchmarking atlas‐level data integration in single‐cell genomics. Nat Methods 19: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM et al (2015) Highly parallel genome‐wide expression profiling of individual cells using nanoliter droplets. Cell 161: 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga Z, Nirmal AJ, Ericson NG, Boswell SA, U'Ren L, Podyminogin R, Chow J, Chen Y‐A, Chen AA, Weinstock DM et al (2021) Micro‐region transcriptomics of fixed human tissue using Pick‐Seq. bioRxiv 10.1101/2021.03.18.431004 [PREPRINT] [DOI]
- Mangiameli S, Chen H, Earl AS, Dobkin J, Lesman D, Buenrostro J, Chen F (2023) Photoselective sequencing: microscopically‐guided genomic measurements with subcellular resolution. Nat Methods 20: 686–694 [DOI] [PubMed] [Google Scholar]
- Marconato L, Palla G, Yamauchi KA, Virshup I, Heidari E, Treis T, Toth M, Shrestha RB, Vöhringer H, Huber W et al (2023) SpatialData: an open and universal data framework for spatial omics. bioRxiv 10.1101/2023.05.05.539647 [PREPRINT] [DOI] [PubMed]
- Marx V (2021) Method of the Year: spatially resolved transcriptomics. Nat Methods 18: 9–14 [DOI] [PubMed] [Google Scholar]
- Mateo LJ, Murphy SE, Hafner A, Cinquini IS, Walker CA, Boettiger AN (2019) Visualizing DNA folding and RNA in embryos at single‐cell resolution. Nature 568: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CT, Lu X, Mehta AS, Angel PM, Drake RR (2023) Applications and continued evolution of glycan imaging mass spectrometry. Mass Spectrom Rev 42: 674–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Shaik S, Devireddy R, Gartia MR (2018) Single‐cell analysis using hyperspectral imaging modalities. J Biomech Eng 140, 0208021–02080216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengwei H, Yang B, Yubao C, Radda JSD, Chen Y, Liu M, Wang S (2020) ProbeDealer is a convenient tool for designing probes for highly multiplexed fluorescence in situ hybridization. Sci Rep 10: 22031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, Hoang M, Jung J, Liang Y, McKay‐Fleisch J et al (2020) Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol 38: 586–599 [DOI] [PubMed] [Google Scholar]
- Moffitt JR, Hao J, Wang G, Chen KH, Babcock HP, Zhuang X (2016) High‐throughput single‐cell gene‐expression profiling with multiplexed error‐robust fluorescence in situ hybridization. Proc Natl Acad Sci USA 113: 11046–11051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JR, Lundberg E, Heyn H (2022) The emerging landscape of spatial profiling technologies. Nat Rev Genet 23: 741–759 [DOI] [PubMed] [Google Scholar]
- Moore J, Basurto‐Lozada D, Besson S, Bogovic J, Brown EM, Burel J‐M, de Medeiros G, Diel EE, Gault D, Ghosh SS et al (2023) OME‐Zarr: a cloud‐optimized bioimaging file format with international community support. Histochem Cell Biol 160: 223–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses L, Pachter L (2022) Publisher correction: museum of spatial transcriptomics. Nat Methods 19: 628 [DOI] [PubMed] [Google Scholar]
- Mulvey CM, Breckels LM, Geladaki A, Britovšek NK, Nightingale DJH, Christoforou A, Elzek M, Deery MJ, Gatto L, Lilley KS (2017) Using hyperLOPIT to perform high‐resolution mapping of the spatial proteome. Nat Protoc 12: 1110–1135 [DOI] [PubMed] [Google Scholar]
- Mund A, Coscia F, Kriston A, Hollandi R, Kovács F, Brunner A‐D, Migh E, Schweizer L, Santos A, Bzorek M et al (2022) Deep visual proteomics defines single‐cell identity and heterogeneity. Nat Biotechnol 40: 1231–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim S‐Y, Choi H, Park Y‐G, Park J‐Y, Hubbert A et al (2015) Simple, scalable proteomic imaging for high‐dimensional profiling of intact systems. Cell 163: 1500–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran M, Riordan DP (2018) Automated cell type classification in intact tissues by single. Elife 7: e30510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HQ, Chattoraj S, Castillo D, Nguyen SC, Nir G, Lioutas A, Hershberg EA, Martins NMC, Reginato PL, Hannan M et al (2020) 3D mapping and accelerated super‐resolution imaging of the human genome using in situ sequencing. Nat Methods 17: 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichterwitz S, Chen G, Aguila Benitez J, Yilmaz M, Storvall H, Cao M, Sandberg R, Deng Q, Hedlund E (2016) Laser capture microscopy coupled with Smart‐seq2 for precise spatial transcriptomic profiling. Nat Commun 7: 12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus M, Soltwisch J, Belov ME, Dreisewerd K (2019) Transmission‐mode MALDI‐2 mass spectrometry imaging of cells and tissues at subcellular resolution. Nat Methods 16: 925–931 [DOI] [PubMed] [Google Scholar]
- Nir G, Farabella I, Pérez Estrada C, Ebeling CG, Beliveau BJ, Sasaki HM, Lee SD, Nguyen SC, McCole RB, Chattoraj S et al (2018) Walking along chromosomes with super‐resolution imaging, contact maps, and integrative modeling. PLoS Genet 14: e1007872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan M, Karaiskos N, Friedman N, Rajewsky N (2019) Gene expression cartography. Nature 576: 132–137 [DOI] [PubMed] [Google Scholar]
- Orjalo A, Johansson HE, Ruth JL (2011) Stellaris™ fluorescence in situ hybridization (FISH) probes: a powerful tool for mRNA detection. Nat Methods 8: i–ii [Google Scholar]
- Ortiz C, Navarro JF, Jurek A, Märtin A, Lundeberg J, Meletis K (2020) Molecular atlas of the adult mouse brain. Sci Adv 6: eabb3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla G, Fischer DS, Regev A, Theis FJ (2022a) Spatial components of molecular tissue biology. Nat Biotechnol 40: 308–318 [DOI] [PubMed] [Google Scholar]
- Palla G, Spitzer H, Klein M, Fischer D, Schaar AC, Kuemmerle LB, Rybakov S, Ibarra IL, Holmberg O, Virshup I et al (2022b) Squidpy: a scalable framework for spatial omics analysis. Nat Methods 19: 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AD, Alexandrov T (2015) Serial 3D imaging mass spectrometry at its tipping point. Anal Chem 87: 4055–4062 [DOI] [PubMed] [Google Scholar]
- Palmer A, Trede D, Alexandrov T (2016) Where imaging mass spectrometry stands: here are the numbers. Metabolomics 12: 1–3 [Google Scholar]
- Pan C, Cai R, Quacquarelli F, Ghasemigharagoz A, Lourbopoulos A, Matryba P, Plesnila N, Dichgans M, Hellal F, Ertürk A (2016) Shrinkage‐mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13: 859–867 [DOI] [PubMed] [Google Scholar]
- Pareek V, Tian H, Winograd N, Benkovic SJ (2020) Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science 368: 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y‐G, Sohn CH, Chen R, McCue M, Yun DH, Drummond GT, Ku T, Evans NB, Oak HC, Trieu W et al (2018) Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat Biotechnol 37: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Choi W, Tiesmeyer S, Long B, Borm LE, Garren E, Nguyen TN, Tasic B, Codeluppi S, Graf T et al (2021) Cell segmentation‐free inference of cell types from in situ transcriptomics data. Nat Commun 12: 3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim J, Lewy T, Rice CM, Elemento O, Rendeiro AF, Mason CE (2022) Spatial omics technologies at multimodal and single cell/subcellular level. Genome Biol 23: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro M, Martinovic M, Bevilacqua V, Hershberg EA, Rossetti G, Beliveau BJ, Bonnal RJP, Pagani M (2020) OligoMinerApp: a web‐server application for the design of genome‐scale oligonucleotide in situ hybridization probes through the flexible OligoMiner environment. Nucleic Acids Res 48: W332–W339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AC, Chiang ZD, Reginato PL, Mangiameli SM, Murray EM, Yao C‐C, Markoulaki S, Earl AS, Labade AS, Jaenisch R et al (2020) In situ genome sequencing resolves DNA sequence and structure in intact biological samples. Science 371: eaay3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perens J, Salinas CG, Skytte JL, Roostalu U, Dahl AB, Dyrby TB, Wichern F, Barkholt P, Vrang N, Jelsing J et al (2020) An optimized mouse brain Atlas for automated mapping and quantification of neuronal activity using iDISCO+ and light sheet fluorescence microscopy. Neuroinformatics 19: 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petras D, Jarmusch AK, Dorrestein PC (2017) From single cells to our planet‐recent advances in using mass spectrometry for spatially resolved metabolomics. Curr Opin Chem Biol 36: 24–31 [DOI] [PubMed] [Google Scholar]
- Petukhov V, Xu RJ, Soldatov RA, Cadinu P, Khodosevich K, Moffitt JR, Kharchenko PV (2021) Cell segmentation in imaging‐based spatial transcriptomics. Nat Biotechnol 40: 345–354 [DOI] [PubMed] [Google Scholar]
- Player AN, Shen LP, Kenny D, Antao VP, Kolberg JA (2001) Single‐copy gene detection using branched DNA (bDNA) in situ hybridization. J Histochem Cytochem 49: 603–612 [DOI] [PubMed] [Google Scholar]
- Rahimi A, Vale‐Silva LA, Savitski MF, Tanevski J, Saez‐Rodriguez J (2023) DOT: fast cell type decomposition of spatial omics by optimal transport. arXiv [csCE] 10.48550/arXiv.2301.01682 [PREPRINT] [DOI]
- Rajewsky N, Almouzni G, Gorski SA, Aerts S, Amit I, Bertero MG, Bock C, Bredenoord AL, Cavalli G, Chiocca S et al (2020) LifeTime and improving European healthcare through cell‐based interceptive medicine. Nature 587: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Barkley D, França GS, Yanai I (2021) Exploring tissue architecture using spatial transcriptomics. Nature 596: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, Park J, Foox J, Hether T, Warren S et al (2021) The spatial landscape of lung pathology during COVID‐19 progression. Nature 593: 564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righelli D, Weber LM, Crowell HL, Pardo B, Collado‐Torres L, Ghazanfar S, Lun ATL, Hicks SC, Risso D (2022) SpatialExperiment: infrastructure for spatially‐resolved transcriptomics data in R using Bioconductor. Bioinformatics 38: 3128–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ (2019) Slide‐seq: a scalable technology for measuring genome‐wide expression at high spatial resolution. Science 363: 1463–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhanifard SH, Mellis IA, Dunagin M, Bayatpour S, Jiang CL, Dardani I, Symmons O, Emert B, Torre E, Cote A et al (2018) ClampFISH detects individual nucleic acid molecules using click chemistry‐based amplification. Nat Biotechnol 37: 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira‐Clavé X, Jiang S, Bai Y, Zhu B, Barlow G, Bhate S, Coskun AF, Han G, Ho C‐MK, Hitzman C et al (2021) Subcellular localization of biomolecules and drug distribution by high‐definition ion beam imaging. Nat Commun 12: 4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt‐Rosen O, Regev A, Oberdoerffer P, Nawy T, Hupalowska A, Rood JE, Ashenberg O, Cerami E, Coffey RJ, Demir E et al (2020) The Human Tumor Atlas Network: charting tumor transitions across space and time at single‐cell resolution. Cell 181: 236–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarenpää S, Shalev O, Ashkenazy H, de Oliveira‐Carlos V, Lundberg DS, Weigel D, Giacomello S (2022) Spatially resolved host‐bacteria‐fungi interactomes via spatial metatranscriptomics. bioRxiv 10.1101/2022.07.18.496977 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Saka SK, Wang Y, Kishi JY, Zhu A, Zeng Y, Xie W, Kirli K, Yapp C, Cicconet M, Beliveau BJ et al (2019) Immuno‐SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol 37: 1080–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R, Farrell JA, Gennert D, Schier AF, Regev A (2015) Spatial reconstruction of single‐cell gene expression data. Nat Biotechnol 33: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad M, Mungur R, Fiehn O, Kehr J (2005) Metabolic profiling of laser microdissected vascular bundles of Arabidopsis thaliana . Plant Methods 1: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro D, Jackson HW, Raghuraman S, Fischer JR, Zanotelli VRT, Schulz D, Giesen C, Catena R, Varga Z, Bodenmiller B (2017) histoCAT: analysis of cell phenotypes and interactions in multiplex image cytometry data. Nat Methods 14: 873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro D, Sokolov A, Yapp C, Chen Y‐A, Muhlich JL, Hess J, Creason AL, Nirmal AJ, Baker GJ, Nariya MK et al (2022a) MCMICRO: a scalable, modular image‐processing pipeline for multiplexed tissue imaging. Nat Methods 19: 311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro D, Yapp C, Sokolov A, Reynolds SM, Chen Y‐A, Sudar D, Xie Y, Muhlich J, Arias‐Camison R, Arena S et al (2022b) MITI minimum information guidelines for highly multiplexed tissue images. Nat Methods 19: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaus TE, Woo S, Xuan F, Chen X, Yin P (2017) A DNA nanoscope via auto‐cycling proximity recording. Nat Commun 8: 696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueder F, Strauss MT, Hoerl D, Schnitzbauer J, Schlichthaerle T, Strauss S, Yin P, Harz H, Leonhardt H, Jungmann R (2017) Universal super‐resolution multiplexing by DNA exchange. Angew Chem Int Ed Engl 56: 4052–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueder F, Unterauer EM, Ganji M, Jungmann R (2020) DNA‐barcoded fluorescence microscopy for spatial omics. Proteomics 20: e1900368 [DOI] [PubMed] [Google Scholar]
- Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, Chu P, Black S, Demeter J, McIlwain DR et al (2020) Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182: 1341–1359.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Sim Y, Kim J, Kim H, Cho I, Nam H, Yoon Y‐G, Chang J‐B (2022) PICASSO allows ultra‐multiplexed fluorescence imaging of spatially overlapping proteins without reference spectra measurements. Nat Commun 13: 2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lubeck E, Schwarzkopf M, He T‐F, Greenbaum A, Sohn CH, Lignell A, Choi HMT, Gradinaru V, Pierce NA et al (2016) Single‐molecule RNA detection at depth by hybridization chain reaction and tissue hydrogel embedding and clearing. Development 143: 2862–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Lubeck E, Zhou W, Cai L (2017) Editorial note to: in situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 94: 745–746 [DOI] [PubMed] [Google Scholar]
- Sountoulidis A, Liontos A, Nguyen HP, Firsova AB, Fysikopoulos A, Qian X, Seeger W, Sundström E, Nilsson M, Samakovlis C (2020) SCRINSHOT enables spatial mapping of cell states in tissue sections with single‐cell resolution. PLoS Biol 18: e3000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraggins JM, Rizzo DG, Moore JL, Noto MJ, Skaar EP, Caprioli RM (2016) Next‐generation technologies for spatial proteomics: integrating ultra‐high speed MALDI‐TOF and high mass resolution MALDI FTICR imaging mass spectrometry for protein analysis. Proteomics 16: 1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, Giacomello S, Asp M, Westholm JO, Huss M et al (2016) Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353: 78–82 [DOI] [PubMed] [Google Scholar]
- Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP (2012) Multi‐isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481: 516–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickels RR, Murray E, Kumar P, Li J, Marshall JL, Di Bella DJ, Arlotta P, Macosko EZ, Chen F (2021) Highly sensitive spatial transcriptomics at near‐cellular resolution with Slide‐seqV2. Nat Biotechnol 39: 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM (2001) Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med 7: 493–496 [DOI] [PubMed] [Google Scholar]
- Su J‐H, Zheng P, Kinrot SS, Bintu B, Zhuang X (2020) Genome‐scale imaging of the 3D organization and transcriptional activity of chromatin. Cell 182: 1641–1659.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhu J, Zhou X (2020) Statistical analysis of spatial expression patterns for spatially resolved transcriptomic studies. Nat Methods 17: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson V, Teichmann SA, Stegle O (2018) SpatialDE: identification of spatially variable genes. Nat Methods 15: 343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáts Z, Wiseman JM, Gologan B, Cooks RG (2004) Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306: 471–473 [DOI] [PubMed] [Google Scholar]
- Takei Y, Zheng S, Yun J, Shah S, Pierson N, White J, Schindler S, Tischbirek CH, Yuan G‐C, Cai L (2021a) Single‐cell nuclear architecture across cell types in the mouse brain. Science 374: eabj1966 [DOI] [PubMed] [Google Scholar]
- Takei Y, Yun J, Zheng S, Ollikainen N, Pierson N, White J, Shah S, Thomassie J, Suo S, Eng C‐HL et al (2021b) Integrated spatial genomics reveals global architecture of single nuclei. Nature 590: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Yang Y, White J, Yun J, Prasad M, Ombelets LJ, Schindler S, Cai L (2023) High‐resolution spatial multi‐omics reveals cell‐type specific nuclear compartments. bioRxiv 10.1101/2023.05.07.539762 [PREPRINT] [DOI]
- Tanevski J, Nguyen T, Truong B, Karaiskos N, Ahsen ME, Zhang X, Shu C, Xu K, Liang X, Hu Y et al (2020) Gene selection for optimal prediction of cell position in tissues from single‐cell transcriptomics data. Life Sci Alliance 3: e202000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanevski J, Flores ROR, Gabor A, Schapiro D, Saez‐Rodriguez J (2022) Explainable multiview framework for dissecting spatial relationships from highly multiplexed data. Genome Biol 23: 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM et al (2017) A subcellular map of the human proteome. Science 356: eaal3321 [DOI] [PubMed] [Google Scholar]
- Tropini C, Earle KA, Huang KC, Sonnenburg JL (2017) The gut microbiome: connecting spatial organization to function. Cell Host Microbe 21: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A et al (2015) Tissue‐based map of the human proteome. Science 347: 1260419 [DOI] [PubMed] [Google Scholar]
- Vandereyken K, Sifrim A, Thienpont B, Voet T (2023) Methods and applications for single‐cell and spatial multi‐omics. Nat Rev Genet 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana MP, Chen J, Knijnenburg TA, Vasan R, Yan C, Arakaki JE, Bailey M, Berry B, Borensztejn A, Brown EM et al (2023) Integrated intracellular organization and its variations in human iPS cells. Nature 613: 345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari M, Mirzazadeh R, Nilsson A, Shariatgorji R, Bjärterot P, Larsson L, Lee H, Nilsson M, Foyer J, Ekvall M et al (2023) Spatial multimodal analysis of transcriptomes and metabolomes in tissues. bioRxiv 10.1101/2023.01.26.525195 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Vickovic S, Eraslan G, Salmén F, Klughammer J, Stenbeck L, Schapiro D, Äijö T, Bonneau R, Bergenstråhle L, Navarro JF et al (2019) High‐definition spatial transcriptomics for in situ tissue profiling. Nat Methods 16: 987–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickovic S, Lötstedt B, Klughammer J, Mages S, Segerstolpe Å, Rozenblatt‐Rosen O, Regev A (2022) SM‐Omics is an automated platform for high‐throughput spatial multi‐omics. Nat Commun 13: 795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FC, Stegle O, Velten B (2023) FISHFactor: a probabilistic factor model for spatial transcriptomics data with subcellular resolution. Bioinformatics 39: btad183, 10.1093/bioinformatics/btad183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang L‐C, Bui S, Nielson A, Wu X, Vo H‐T, Ma X‐J, Luo Y (2012) RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J Mol Diagn 14: 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Su J‐H, Beliveau BJ, Bintu B, Moffitt JR, Wu C‐T, Zhuang X (2016) Spatial organization of chromatin domains and compartments in single chromosomes. Science 353: 598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Woehrstein JB, Donoghue N, Dai M, Avendaño MS, Schackmann RCJ, Zoeller JJ, Wang SSH, Tillberg PW, Park D et al (2017) Rapid sequential in situ multiplexing with DNA exchange imaging in neuronal cells and tissues. Nano Lett 17: 6131–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Moffitt JR, Zhuang X (2018a) Multiplexed imaging of high‐density libraries of RNAs with MERFISH and expansion microscopy. Sci Rep 8: 4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J et al (2018b) Three‐dimensional intact‐tissue sequencing of single‐cell transcriptional states. Science 361: eaat5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zeng Y, Saka SK, Xie W, Goldaracena I, Kohman RE, Yin P, Church GM (2020) Multiplexed in situ protein imaging using DNA‐barcoded antibodies with extended hybridization chain reactions. bioRxiv 10.1101/274456 [PREPRINT] [DOI] [PMC free article] [PubMed]
- Wang G, Heijs B, Kostidis S, Mahfouz A, Rietjens RGJ, Bijkerk R, Koudijs A, van der Pluijm LAK, van den Berg CW, Dumas SJ et al (2022a) Analyzing cell‐type‐specific dynamics of metabolism in kidney repair. Nat Metab 4: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xing X, Zeng X, Jackson SR, TeSlaa T, Al‐Dalahmah O, Samarah LZ, Goodwin K, Yang L, McReynolds MR et al (2022b) Spatially resolved isotope tracing reveals tissue metabolic activity. Nat Methods 19: 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Chen Z, Shi L, Long R, Anzalone AV, Zhang L, Hu F, Yuste R, Cornish VW, Min W (2017) Super‐multiplex vibrational imaging. Nature 544: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JA, Regev A, Zhang F (2019) DNA microscopy: optics‐free spatio‐genetic imaging by a stand‐alone chemical reaction. Cell 178: 229–241.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G, Leen V, Rohand T, Sauer M, Hofkens J (2023) Current progress in expansion microscopy: chemical strategies and applications. Chem Rev 123: 3299–3323 [DOI] [PubMed] [Google Scholar]
- Xia C, Babcock HP, Moffitt JR, Zhuang X (2019a) Multiplexed detection of RNA using MERFISH and branched DNA amplification. Sci Rep 9: 7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Fan J, Emanuel G, Hao J, Zhuang X (2019b) Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle‐dependent gene expression. Proc Natl Acad Sci USA 116: 19490–19499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapp C, Novikov E, Jang W‐D, Chen Y‐A, Cicconet M, Maliga Z, Jacobson CA, Wei D, Santagata S, Pfister H et al (2022) UnMICST: Deep learning with real augmentation for robust segmentation of highly multiplexed images of human tissues. Commun Biol 5: 1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Bar‐Joseph Z (2022) GCNG: graph convolutional networks for inferring gene interaction from spatial transcriptomics data. Genome Biol 21: 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh JN, Steenberg CD, Bois JS, Wolfe BR, Pierce MB, Khan AR, Dirks RM, Pierce NA (2011) NUPACK: analysis and design of nucleic acid systems. J Comput Chem 32: 170–173 [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen D, Song D, Liu X, Zhang Y, Xu X, Wang X (2022) Clinical and translational values of spatial transcriptomics. Signal Transduct Target Ther 7: 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Deng Y, Kukanja P, Agirre E, Bartosovic M, Dong M, Ma C, Ma S, Su G, Bao S et al (2023) Spatial epigenome–transcriptome co‐profiling of mammalian tissues. Nature 616: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, Bordbar D, Shan D, Samanamud J, Mahajan A et al (2019) Immune and genomic correlates of response to anti‐PD‐1 immunotherapy in glioblastoma. Nat Med 25: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E, Stone MR, Ren X, Guenthoer J, Smythe KS, Pulliam T, Williams SR, Uytingco CR, Taylor SEB, Nghiem P et al (2021) Spatial transcriptomics at subspot resolution with BayesSpace. Nat Biotechnol 39: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Piehowski PD, Zhao R, Chen J, Shen Y, Moore RJ, Shukla AK, Petyuk VA, Campbell‐Thompson M, Mathews CE et al (2018a) Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat Commun 9: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Dou M, Piehowski PD, Liang Y, Wang F, Chu RK, Chrisler WB, Smith JN, Schwarz KC, Shen Y et al (2018b) Spatially resolved proteome mapping of laser capture microdissected tissue with automated sample transfer to nanodroplets. Mol Cell Proteomics 17: 1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Li Q, Liao T, Yin X, Chen Q, Wang Z, Dai M, Yi L, Ge S, Miao C et al (2021) Metabolomic profiling of single enlarged lysosomes. Nat Methods 18: 788–798 [DOI] [PubMed] [Google Scholar]
- Zhuang X (2021) Spatially resolved single‐cell genomics and transcriptomics by imaging. Nat Methods 18: 18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]


