Abstract
Objectives
Systematic review of SARS-CoV-2 seroprevalence studies undertaken in the WHO European Region to measure pre-existing and cumulative seropositivity prior to the roll out of vaccination programmes.
Design
A systematic review of the literature.
Data sources
We searched MEDLINE, EMBASE and the preprint servers MedRxiv and BioRxiv in the WHO ‘COVID-19 Global literature on coronavirus disease’ database using a predefined search strategy. Articles were supplemented with unpublished WHO-supported Unity-aligned seroprevalence studies and other studies reported directly to WHO Regional Office for Europe and European Centre for Disease Prevention and Control.
Eligibility criteria
Studies published before the widespread implementation of COVID-19 vaccination programmes in January 2021 among the general population and blood donors, at national and regional levels.
Data extraction and synthesis
At least two independent researchers extracted the eligible studies; a third researcher resolved any disagreements. Study risk of bias was assessed using a quality scoring system based on sample size, sampling and testing methodologies.
Results
In total, 111 studies from 26 countries published or conducted between 1 January 2020 and 31 December 2020 across the WHO European Region were included. A significant heterogeneity in implementation was noted across the studies, with a paucity of studies from the east of the Region. Sixty-four (58%) studies were assessed to be of medium to high risk of bias. Overall, SARS-CoV-2 seropositivity prior to widespread community circulation was very low. National seroprevalence estimates after circulation started ranged from 0% to 51.3% (median 2.2% (IQR 0.7–5.2%); n=124), while subnational estimates ranged from 0% to 52% (median 5.8% (IQR 2.3%–12%); n=101), with the highest estimates in areas following widespread local transmission.
Conclusions
The low levels of SARS-CoV-2 antibody in most populations prior to the start of vaccine programmes underlines the critical importance of targeted vaccination of priority groups at risk of severe disease, while maintaining reduced levels of transmission to minimise population morbidity and mortality.
Keywords: epidemiology, public health, infectious diseases, COVID-19, systematic review
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study provides a comprehensive systematic review of SARS-CoV-2 seroprevalence literature of all languages and unpublished data.
Thorough literature search of major electronic databases and reporting as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Due to heterogeneity between studies including sampling frame, population and stage of epidemic at time of serosurvey results are described narratively.
Seroprevalence may be underestimated as antibody waning was not taken into account.
Introduction
The novel virus, SARS-CoV-2, was first identified in Wuhan, China in December 2019 and spread rapidly around the world. At that time, the transmissibility, population susceptibility, clinical spectrum and infection severity of this novel virus were all unknown. By 1 January 2021, approximately 83 million confirmed cases were reported globally, while in the WHO European Region, there were 4.9 million cases.1 However, notified cases and deaths are an underestimate of the true number of infections for reasons including clinical presentation with a large proportion of asymptomatic or mildly symptomatic cases, testing and reporting strategies and healthcare seeking behaviour.2 Asymptomatic infection has been reported in many studies with the proportion ranging from 6% to 41%3–5 so a significant proportion of SARS-CoV-2 infections will be missed through case-based surveillance systems.6
Seroprevalence studies, which measure SARS-CoV-2 antibodies, can provide an important complement to routine surveillance, particularly as part of the assessment of novel emerging respiratory pathogens. Seroprevalence surveys are essential to assess the true extent of prevalence of pre-existing cross-reactive antibodies in the population; to measure population age-specific and geographical cumulative seroincidence as the novel virus spreads and to contribute to estimating infection severity. As the majority of SARS-CoV-2-infected individuals have a detectable humoral immune response on average 10–14 days after symptom onset and most individuals seroconvert within 3–4 weeks of infection,7 and anti-SARS-CoV-2 antibodies are predictive of immune protection,8 9 seroprevalence studies can provide an indication of population levels of humoral immunity and inform public health policies.
Since the start of the COVID-19 pandemic, there has been a rapid accumulation of seroepidemiological studies describing the seroprevalence of SARS-CoV-2. This review aims to provide a comprehensive review of studies conducted in the WHO European Region between 1 January 2020 and 31 December 2020 in the general population, with the aim to synthesise evidence on the extent of transmission across the region and population immunity to this newly emerging infection before the start of the COVID-19 vaccination programmes. As SARS-CoV-2 continues to circulate, understanding the age-specific population seropositivity remains critical for policymakers and public health officials to make informed decisions on optimal public health interventions.10
Methods
Search strategy
We searched MEDLINE, WHO COVID, EMBASE and the preprint servers medRxiv and bioRxiv within the WHO ‘COVID-19 Global literature on coronavirus disease’ database on 21 October 2020 and 12 January 2021. The searches spanned the period 1 January 2020–31 December 2020 and was not restricted by language. We supplemented these articles with WHO-supported Unity seroprevalence studies and unpublished studies reported to WHO Regional Office for Europe and European Centre for Disease Prevention and Control (ECDC). The selection process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 The full search strategy, search terms as well as inclusion and exclusion criteria are described in online supplemental material 1.
bmjopen-2022-064240supp001.pdf (120.7KB, pdf)
Data extraction
We combined the references from all databases, removed duplicates and imported the remaining articles into Rayyan software12 for screening of titles and abstracts according to the inclusion and exclusion criteria (online supplemental table S1). After the initial screening of title and abstracts, two independent researchers assessed full-text publications for eligibility. Data from preprint articles were extracted and later replaced with data from published articles, where necessary. At least two independent researchers extracted the eligible studies; a third researcher resolved any disagreements on assessment of eligibility or extraction. We extracted the following data: first author, publication date, country, region, period of study, population type, population age, sampling method, sample size, laboratory methods used, confirmatory testing, test performance, crude and adjusted point seroprevalence estimates, antibody type and analysis methodology.13–128 Comparison was made with weekly laboratory-confirmed case and death reports.
Study quality assessment
We used a modified Joana Briggs quality assessment scoring system to assess the overall risk of bias of each study.129 The criteria included: (a) the sampling frame (to assess representativeness of the general population); (b) stratification (age, sex or population); (c) recruitment method (random, convenience), (d) adequacy of sample size, (e) serological methods and validation; (f) and statistical analyses (adjustment of results to account for the sensitivity and specificity of the test). A cumulative quality score classified the overall risk of bias of each study into high risk of bias (1-3), medium risk of bias (4-6) or low risk of bias (>6). Two independent researchers conducted the quality assessment; a third researcher resolved any disagreements. See online supplemental table S2 for more details on the quality criteria. For the purposes of quality assessment, the threshold for acceptable test performance was ≥95% sensitivity and >97% specificity for laboratory assays and ≥90% sensitivity and >97% specificity for point-of-care tests.130
Data analysis
We used descriptive statistics to summarise estimates by subgroup (median and IQR). We generated forest plots to display the data and explore variations according to specific characteristics, including time, geographical location and population group. Correlation between cumulative incidence and cumulative deaths and seroprevalence estimates from studies of the general population was explored using Spearman’s rank correlation. We compared seroprevalence estimates from studies of the general population and the cumulative incidence and deaths at the start of each study. Analyses were performed in Microsoft Excel (V.2016) and R V.4.0.4.
Patient and public involvement
No patient involved.
Results
Literature search
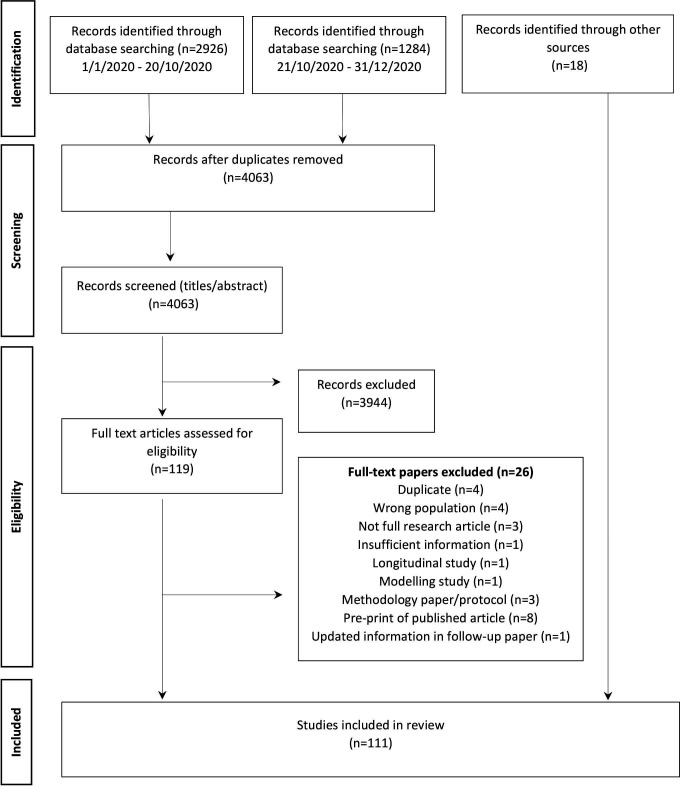
The literature search resulted in 4063 studies. After deduplication, application of inclusion and exclusion criteria and supplementation with articles from other sources, a total of 111 studies were included in this review. Of these, 77 were published articles, 19 were preprints, 9 were institutional report and 6 were studies were identified through reporting of unpublished results to WHO or ECDC. See figure 1 PRISMA flow diagram study selection.
Figure 1.
PRISMA flow chart of SARS-CoV-2 seroprevalence study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics
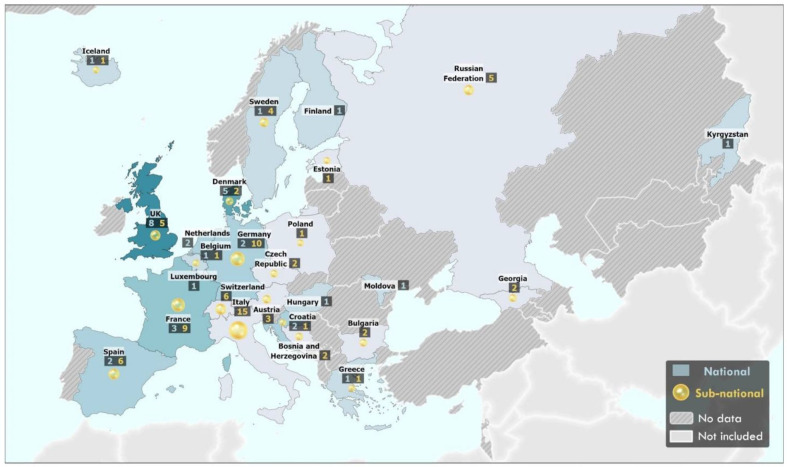
The 111 studies included 224 seroprevalence estimates from 26 of the 53 countries in the WHO European Region (figure 2). The majority of studies (n=82; 74%) were conducted in 19 EuropeanUnion/European Economic Area (EU/EEA) countries, while 29 studies (26%) conducted in 7 non-EU/EEA countries (Bosnia and Herzegovina, Georgia, Kyrgyzstan, Republic of Moldova, Russian Federation, Switzerland and the UK) (figure 2; table 1). Fifty-six (50%) studies were aligned with the WHO Unity population-based seroepidemiological investigation criteria related to study design, data collection and analysis.131 The majority of studies (n=69, 62%) used non-random or convenience sampling of the population. Forty-one (37%) studies used random sampling, while one study did not report sampling methodology. Characteristics and details of included studies are shown in table 1 and online supplemental table S1, respectively.
Figure 2.
Geographical distribution of SARS-CoV-2 seroprevalence studies published in the WHO European Region between 1 January2020 and 31 December 2020. Countries with national-level seroprevalence studies are reported in blue (shade of blue reflects the number of studies conducted in the country/territory). Subnational-level seroprevalence studies are reported as a yellow circle (size of circle reflects number of subnational studies conducted in the country/territory). A number of studies are listed in boxes under name. Countries with not studies are coloured in grey. The designations employed and the presentation of this material do not imply the expression of any opinion whatsoever on the part of the secretariat of the WHO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries. Dotted and dashed lines on maps represent approximate locations for which there may not yet be full agreement.
Table 1.
Study characteristics
| Characteristics | No of studies | % |
| Total | 111 | 100 |
| Study characteristics | ||
| Country | ||
| WHO European Region (EU/EEA*) | 82 | 74 |
| WHO European Region (outside of EU/EEA) | 29 | 26 |
| WHO UNITY alignment | ||
| Unity-aligned | 56 | 50 |
| Not unity-aligned | 55 | 50 |
| Publication type | ||
| Peer-reviewed article | 77 | 69 |
| Preprint | 19 | 17 |
| Institutional report | 9 | 8 |
| Not yet published | 6 | 5 |
| Geographical level | ||
| National | 33 | 30 |
| Regional | 27 | 24 |
| City/local | 50 | 44 |
| Multiple | 1 | 1 |
| Sampling strategy | ||
| Convenience | 69 | 62 |
| Random | 41 | 37 |
| Not reported | 1 | 1 |
| Population type | ||
| Household/community | 45 | 41 |
| Residual sera | 13 | 12 |
| Blood donors | 16 | 14 |
| Patients seeking healthcare (non-COVID-19) | 13 | 12 |
| Pregnant or parturient women | 7 | 6 |
| Other/multiple | 23 | 21 |
| Quality assessment | ||
| Low risk of bias | 41 | 37 |
| Medium risk of bias | 40 | 36 |
| High risk of bias | 24 | 22 |
| N/A | 6 | 5 |
| Sample size | ||
| <1000 | 45 | 41 |
| ≥1000 | 66 | 59 |
| Laboratory characteristics | ||
| Serological method | ||
| ELISA | 55 | 50 |
| CMIA/CLIA | 42 | 38 |
| LFA | 25 | 23 |
| MN | 10 | 9 |
| Other | 8 | 7 |
| Not reported | 2 | 1 |
| Type of assay | ||
| Commercial | 90 | 81 |
| In-house | 26 | 23 |
| Not reported | 2 | 1 |
*EU/EEA:EuropeanUnion/EuropeanEconomicArea
CLIA, chemiluminescent immunoassay; CMIA, chemiluminescence microparticle immunoassay; LFA, lateral flow immunoassay; MN, microneutralisation assay; N/A, not available.
In total, 72 (65%) of the studies provided representative estimates from the general population, of which sample frames included 45 (41%) studies of household or community samples, 13 (12%) residual sera, 13 (12%) patients seeking healthcare for non-COVID-19-related issues, 7 (6%) pregnant or parturient women. Sixteen (14%) studies sampled blood donors as a proxy for the general population while 23 (21%) sampled other or multiple populations. Studies were conducted at differing geographical levels within a country, including at the national level (n=33; 30%), regional level (n=27; 24%) and city or local level (n=50; 44%). One study reported both national and regional estimates.
Over half of the studies used one serological assay (71; 67%) while 34 (31%) used at least two different assays. In 82 studies (74%), commercial assays from various sources were used, 20 (18%) studies used an in-house assay only and 6 studies (5%) used both a commercial and in-house developed assay. The test method was not reported in two studies. An ELISA was the method most commonly employed (n=55, 50%), followed by chemiluminescent immunoassay or chemiluminescence microparticle immunoassay (n=42, 38%) and lateral flow immunoassays (LFAs) (n=25, 23%). Seventeen studies (15%) used LFAs exclusively. Ten studies (9%) employed in-house microneutralisation assays to assess the neutralising ability of SARS-CoV-2 antibodies.
Of 90 studies that used a commercial assay, 33 studies (37%) reported the use of tests with acceptable sensitivity and specificity. Of those that independently validated assay performance (n=41, 46%), 14 (34%) reported acceptable sensitivity and specificity, while 27 (66%) did not meet these thresholds. Of the 20 studies that used an in-house assay, 9 (45%) reported an acceptable test performance, 4 (20%) performed below these thresholds and 7 (35%) did not report on test performance. The majority of studies (n=83, 75%) did not report adjustment for test sensitivity or specificity in their analysis.
Based on our quality scoring system (online supplemental table S2), 81 studies (73%) were of high or medium quality reflecting a low or medium risk of bias, respectively (medium quality: n=40, 36%; high quality n=41, 37%) (online supplemental table S3). A total of 24 studies (22%) were determined to be at high risk of bias, largely due to non-random sampling frame, weak representativeness of the general population or lack of adjustment for sampling bias or test performance.
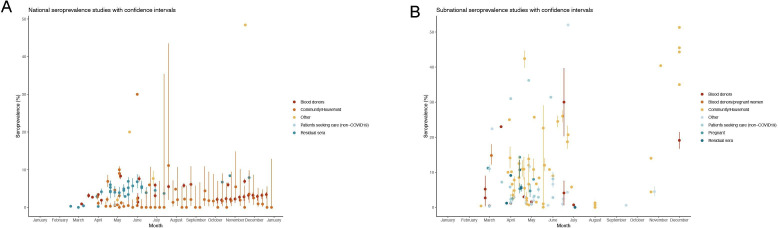
Seroprevalence estimates
Seroprevalence estimates (n=88) from national studies ranged from 0% (95% CI 0.0% to 0.7%) in Finland in May23 to 51.3% in Georgia in December25 (median 2.2% (IQR 0.7%–5.2%); n=124) (figure 3A), while seroprevalence estimates from studies spanning regions, cities or towns (n=101) ranged from 0% (95% CI 0.0% to 0.5%) in Czech Republic in August 202025 to 52% in a Médecins Sans Frontières centre in Paris, France during an outbreak with widespread community transmission in June 2020111 (median 5.8% (IQR 2.3%–12%); n=101) (figure 3B).
Figure 3.
National (A) and subnational (B) seroprevalence estimates of SARS-CoV-2 antibodies over time in the WHO European Region (1 January 2020–31 December 2021).
A total of 45 studies provided seroprevalence estimates (n=105) from community or household samples and 39 studies (87%) were found to be of high or medium quality. Seroprevalence estimates ranged from 0% (95% CI 0% to 0.7%) in Finland in May and to 51.3% in December 2020 in Georgia25 (median 2.6% (IQR 0.5%–10%) n=105) (online supplemental figure S1).
Thirteen studies screened residual clinical samples26–39 between February and November 2020, of which 9 (70%) were of high or medium quality. Seroprevalence estimates (n=34) in this population varied across countries ranging from 0% (95% CI 0% to 0.23%) in Greece in March to 18.7% (95% CI 16.7% to 23.3%) in Sweden in June (median 4.5% (IQR 3.5%–5.9%); n=34) (online supplemental figure S2A).
Eighteen studies (17%) used blood donors as a proxy for the general population between February and December 2020, of which 16 were of high or medium quality. Seroprevalence estimates (n=42) in blood donors varied across countries, ranging from 0.4% in Germany between March and June73 to 30% in Tensta (Stockholm) following a period of high incidence in June78 (median 5.8% (IQR 2.1%–5.7%) n=42) (online supplemental figure S2B).
Eight studies investigated the seroprevalence of SARS-CoV-2 in pregnant or parturient women, reporting estimates ranging from 2.6% (95% CI 1.7% to 4%) and 14.3% between March and June 2020 (median 6.9% (IQR 5.1%–12%); n=8)99–105 (online supplemental figure S2C). One study provided combined estimates of blood donors and pregnant women of 14.8% in Sweden between March and December.127
Fourteen studies provided 16 estimates from individuals seeking healthcare for non-COVID-19-related reasons and seven (50%) of these were medium or high quality. Estimates ranged from 0.3% in Zurich, Switzerland in March126 to 36.2% in London in April97 (median 4.1% (IQR 2.1%–8.8%); n=16) from March to August 2020. The highest seroprevalence estimates (>10%) in this group were observed in three patient groups investigated following local widespread community transmission, oncology patients (31%) in Bergamo, Italy in April 2020,89 oncology patients (31.4%) in Madrid between May and June 202095 and haemodialysis patients (36.2%) in London in April and May 202097 and patients (38.5%) in Barcelona, Spain in April125 (online supplemental figure S2D).
Forty-four (41%) studies reported seroprevalence estimates stratified by age. Seroprevalence estimates varied considerably across age groups and estimates tended to be lower in children (<18 years)36 38 49 and older age groups (>60 years).33 41 47 49 66 67 132 While a number of studies reported a high seroprevalence in older age groups (>55 years),26 32 33 41 70 75 94 122 some studies also reported a higher seroprevalence in younger age groups (<40 years).38 50 70 77 In studies that reported seroprevalence estimates by sex, similar seroprevalence results were observed between females and males with the exception of a study in Italy,94 Russian Federation43 and Kyrgyzstan36 which each found a higher seroprevalence in females.
Seroprevalence estimates over time
A number of studies provided seroprevalence estimates prior to, or at the early stages of the epidemic in the country (online supplemental figure S3). Of these, overall study estimates were largely below 10%, however higher seroprevalence was noted in a number of population-specific, regional or local studies,13 28 29 32 33 89 108 110 with suggestion of earlier undetected transmission in some countries.36 104 116 127 A total of 16 studies reported seroprevalence estimates spanning multiple timepoints or stages of the epidemic.20 23 25 46 49 50 52 55 58 61 62 65 76 79–84 113 117 120 125 126 In a serial cross-sectional study in France,58 residual blood sampled before, during and after a national lockdown showed a seroprevalence of 0.41%, 4.14% and 4.93%, respectively. In Georgia, in a community sample, an increase in seroprevalence from 0%–1.3% in August 2020 to 35%–51.3% in the same regions in December 2020 was noted.25 A seroprevalence study in blood donors conducted in Milan between February and April 2020 during a period of intense transmission found an increase in seroprevalence from 2.7% (95% CI 0.3% to 6.0%) to 5.2% (95% CI 2.4% to 9.0%), with an adjusted rate of increase in antibodies (IgG) of 2.7%±1.3% per week as social distancing measures were gradually implemented.76 While in Finland, weekly testing of blood donors from April 2020 onwards showed a consistently low seroprevalence in the general population over time (0.28% (95% CI 0.05% to 1.55%) in early April 2020 to 0% (95% CI 0% to 12.87%) in late December 2020.64
Correlation between seroprevalence and cumulative incidence
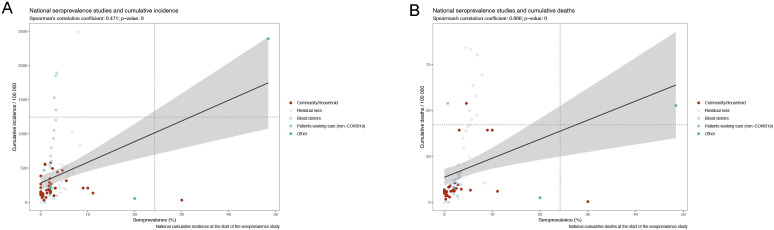
The relationship between seroprevalence and reported SARS-CoV-2 laboratory-confirmed cumulative case and deaths incidence was also explored. While seroprevalence from national studies correlated moderately with cumulative incidence (Spearman’s rank correlation coefficient, 0.471) (figure 4A), a stronger correlation was observed between seroprevalence estimates and cumulative SARS-CoV-2 deaths (Spearman’s rank correlation coefficient, 0.666) (figure 4B).
Figure 4.
Correlation between seroprevalence point estimates from low to medium risk of bias studies and cumulative (A) incidence and (B) deaths in all populations, in the WHO European Region (1 january 2020–31 December 2020).
Discussion
In this study, we report the results of 111 studies, including 224 seroprevalence estimates from 26 countries in the WHO European Region undertaken until December 2020, prior to the implementation of national COVID-19 vaccine campaigns. A large variation in study methodologies was noted across the studies, with an over-representation of studies from high-income countries in Western Europe.
Overall, population-wide seroprevalence estimates were low (below 10%) across the Region early in 2020 before the onset of widespread community transmission and remained low across the Region throughout 2020, despite circulation of SARS-CoV-2 over this period. Higher estimates were observed at a regional or local level in populations that had experienced intense community transmission (up to 52%). Furthermore, a positive correlation between seroprevalence estimates and national cumulative incidence was observed, with a stronger correlation between seroprevalence and cumulative mortality.
The wide variation in seroprevalence estimates across the region are likely to reflect many factors including the differences in the population studied, local stage of the epidemic and the public health and social measures implemented in response to the epidemic at that time. The general low seroprevalence both at the start of the pandemic and at the end of 2020 is in line with a number of global systematic review conducted to date133–136 and together indicates that the majority of the proportion of the population in the WHO European Region were and remain susceptible to infection 1 year after the identification of SARS-CoV-2 and prior to the start of national vaccination campaigns. In a global systematic review, Chen et al estimated a seroprevalence of 4.2% (2.7%–5.8%) across the European Region until August 2020135 while Rostami et al estimated a pooled prevalence of 3.17% (1.96%–4.38%), 4.41% (2.20%–6.61%), 5.27% (3.97%–6.57%) in Western, Southern and Northern Europe, respectively.134 In the same period, Bobrovitz et al reported a pooled estimate of 1.6% (1.1%–5.2%) seroprevalence in studies conducted across Central Europe, Eastern Europe and Central Asia137 and 12.2% (4.5%–25.4%) from population-wide studies conducted until December 2020.133
A number of studies reported low seroprevalence in younger and older age groups, a finding observed in other systematic reviews.133 135 138 Such findings have important implications, as groups such as the elderly are at higher risk of severe outcome following infection—and lack of cross-protective immunity indicates that all age groups will anticipate seeing high infection attack rates without implementation of measures such as vaccination of priority groups, together with strengthening of public health and social measures to reduce SARS-CoV-2 transmission.
When reviewed alongside case notification data, seroprevalence estimates can provide greater insight into the local evolution of the pandemic. In this review, a positive correlation between seroprevalence estimates and national cumulative incidence in a number of countries was observed, suggesting that seroprevalence is a reflection of the duration and intensity of community transmission. It should be noted, however, that during the initial peak of infections in Europe in the spring of 2020, testing in many countries was not yet optimal and case notification data at this time are unlikely to provide a robust proxy for incidence in many instances. In line with this, several studies found seroprevalence estimates to be higher than the corresponding cumulative incidence of SARS-CoV-2 infections, suggesting a substantial underascertainment of infection through notifications, due to a number of factors including the asymptomatic or mild nature of disease, healthcare seeking behaviour, lack of testing capacity and testing and reporting strategies. Indeed, we also found a stronger association between seroprevalence and cumulative case mortality than cumulative case incidence, providing further evidence to support the suggestion of case underascertainment, as laboratory-confirmed mortality surveillance for COVID-19 is likely to be more comprehensive.
The varying quality of studies in this review reflects the challenge of conducting seroepidemiological studies of high quality. Indeed, this review found that only 50% of all studies undertaken in the WHO European region in 2020 were aligned with the WHO Unity study initiative. Few of the national (n=5; 15%) or regional (n=2; 7%) studies were determined to be of high risk of bias, while 17 (34%) of studies conducted at a local level (cities or towns) were graded as such. This variation may be explained by the level of resources and epidemiological support available to studies conducted at the regional or national level.
The majority of studies identified in this review used convenience rather than random sampling, which may have reduced the true representativeness of the estimates derived, though such convenience sampling is likely to provide a good estimate of population exposure for widely circulating viral infections. Many studies also included individuals that were not fully representative of the population under study, which may have introduced bias. For example, this review included studies that explored seroprevalence in the general population by using various proxy populations such as blood donors and residual blood. Blood donors are known to differ from the general population in that they are often a young, healthy adult population selected on the basis of lack of recent infection139 and seroprevalence may, therefore, be over or underestimated in this group. Residual sera, on the other hand, derives from individuals who have sought healthcare and may therefore have pre-existing comorbidities or be at higher risk of SARS-CoV-2 infection. However, we found that seroprevalence estimates for these distinct populations are in good agreement with the general population.
We also found that there was a high degree of heterogeneity across serological assays used. The majority of studies used commercial tests of varying sensitivity and specificity to detect SARS-CoV-2 targeted antibodies, although some of these assays have now been shown to have excellent performance.140 141 However, under half of studies performed independent validation of these kits with internal controls and serum panels and only 25% accounted for the sensitivity and specificity of the tests in their statistical analyses. As SARS-CoV-2 serological tests have been found to have variable test performance,140 141 independent validation at local level in combination with use of an WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody has been widely promoted as part of the Solidarity II initiative.142 143 Other options include the Joint Research Centre144 reference materials for the quality control of SARS-CoV-2 antibody tests. Use of these materials will allow for the potential correction for sensitivity and specificity during the statistical analysis, would allow for more robust estimates and greater comparability among countries in the region.
Overall, the findings of this review highlight the need for international collaboration to standardise approaches and support countries in conducting robust comparable studies. WHO, in collaboration with technical partners, has developed the Unity studies,15 90 a global seroepidemiology standardisation initiative for COVID-19, which aims to increase quality evidence-based knowledge in country and regions for action through the availability of standardised seroepidemiology investigation protocols and antibody assays. A primary aim of this global initiative is the provision of direct support to countries to develop country specific protocols, with particular attention provided to low-income and middle-income countries (LMICs), and to support aggregation, comparison and analysis of robust Unity-aligned studies through strong coordination between WHO Country offices, Regional offices and Headquarters. A large proportion of the studies identified in this systematic review were conducted in Western European countries, with a relative scarcity of seroprevalence studies from other countries by the end of 2020, an observation noted in other systematic reviews.133–135 138 This highlights the urgent need for enhanced capacity, the provision of additional support to LMICs and the sharing of information to address the gap in knowledge and tackle research inequity. To counteract the skewedness in the WHO European Region, the WHO Unity protocols have been widely promoted by WHO and ECDC and technical support has been provided to tailor the protocols to local contexts, together with laboratory and financial support to LMICs. In addition, WHO and ECDC jointly established a network of approximately 300 public health professionals to facilitate discussions in related to SARS-CoV-2 seroprevalence, promote timely sharing of results and knowledge and further build capacity in the WHO European Region.
This systematic review comprehensively describes the seroprevalence of SARS-CoV-2 in the first year of the pandemic, prior to the widespread implementation of national vaccine programmes. With the inclusion of as yet unpublished data from LMICs, this review contributes to research equity across Member States income levels and provides a more representative overview of the situation in the WHO European Region than would published studies alone. In addition, we evaluated the UNITY study alignment of studies to assess quality and comparability.
This review has some limitations. First, there was significant heterogeneity among the studies, including sampling frame, population and stage of epidemic at time of serosurvey, which makes comparability across studies difficult. Due to such heterogeneity, we opted to not provide one pooled estimate nor conduct a meta-analysis, as interpretation would be difficult and may not accurately reflect the picture in the WHO European Region. Second, while population-based serological surveys can provide a more accurate estimation of the overall rates of SARS-CoV-2 infection within a population, this approach does not consider antibody waning, which cannot be easily accounted for as antibody levels vary depending on disease severity145 and longevity is expected to vary greatly across SARS-CoV-2-infected individuals.146 In addition, while seroprevalence studies provide an estimate of population exposure, seropositivity is not the only predictor of susceptibility to infection. Finally, due to the rapid accumulation of data related to SARS-CoV-2 seroepidemiology and the advent of the ‘preprint era’, not all included studies have been published and may, therefore, be subject to change on peer review.
Conclusion
As SARS-CoV-2 continues to circulate, understanding the population seropositivity remains critical for policy-makers and public health officials to make informed decisions on optimal public health interventions, such as lifting or tightening of restrictions and targeted vaccination.10 147 In this study, we found evidence that SARS-CoV-2 antibody seroprevalence across the WHO European Region was low prior to widespread circulation and remained low in the general population during 2020. This suggests that much of the population remained susceptible to infection prior to the implementation of national COVID-19 vaccine campaigns from early 2021 onwards. We also found variation in seroprevalence estimates between and within countries during 2020, with evidence of increased prevalence in areas following high levels of transmission and some association with incidence and mortality trends over time. It is clear that antibody-mediated ‘herd immunity’ through natural infection is not attainable in most countries and COVID-19 vaccines should continue to be distributed widely and equitably to protect priority groups and the wider population. Given the issue of antibody waning, all efforts must be also directed towards well-informed and evidence-based implementation and maintenance of non-pharmaceutical interventions at a local and national level to stem any future waves of the pandemic. Indeed, as vaccine programmes continue to be implemented, standardised seroprevalence studies will be instrumental to evaluate both natural and vaccine derived immunity overtime to guide public health actions and decision-making.
Seroprevalence studies have been of great value to COVID-19 pandemic response efforts, providing estimates of the true extent and dynamics of SARS-CoV-2 infection overtime and the lessons identified from COVID-19, in particular the need for standardised global serosurveillance systems, will inform future pandemic preparedness.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Principle Investigators of Unity-aligned studies who have kindly shared their unpublished data: Bosnia and Herzegovina: Dejan Bokonjić (University of East Sarajevo Faculty of Medicine Foča, Bosnia and Herzegovina (Republic of Srpska)), Elma Catovic Baralija (Institute for Transfusion Medicine of the Federation of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina), Sanjin Musa (Institute for Public Health of the Federation of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina (Federation of Bosnia and Herzegovina)), Ranko Škrbić (Medical Faculty in Banja Luka, Bosnia and Herzegovina (Republic of Srpska)); Bulgaria: Angel Kunchev (Ministry of Health of the Republic of Bulgaria), Savina Stoitsova (National Center of Infectious and Parasitic Diseases); Georgia: Olgha Tarkhan-Mouravi (National Center for Disease Control and Public Health Georgia, Head of Vaccine-Preventable and Respiratory Diseases Division, Georgia); Kyrgyzstan: Tatyana Kuchuk (Republican Scientific and Practical Center for Laboratory Diagnostic Quality Control of infectious diseases, Ministry of Health of the Kyrgyz Republic, Kyrgyzstan), Nurmatov Zuridin (Republican Scientific and Practical Center for Control of Viral Infections, Ministry of Health of the Kyrgyz Republic, Kyrgyzstan); Republic of Moldova: Alexei Ceban (National Agency for Public Health, Chișinău, Republic of Moldova). We would also like to thank Céline Roman (WHO EURO), Jeffrey Pires (WHO EURO), Tjede Funk (ECDC) and Tommi Karki (ECDC) for data collection, analysis and visualisation; Tomas Allen and the WHO HQ library team for developing the search strategy; SeroTracker (Mairead Whelan, Zihan Li, Niklas Bobrovitz, Harriet Ware and Rahul K Arora) and WHO HQ colleagues (Hannah Lewis and Brianna Cheng); and all ECDC, WHO Headquarters, Regional and Country Office colleagues who contributed to this work. The authors are alone responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO or ECDC.
Footnotes
Contributors: Conceptualisation: AV, ED, GSF, RP, MK, PP, AN, MV, IB, LS and EB; Data curation: AV, GSF, ED, MK and DSL; Formal analysis: AV, GSF, ED and MK; Investigation: AV, GSF, ED, MK, AN and MV; Methodology: AV, GSF, ED, MK, AN, MV, RP, PP and EB; Guarantor: AV; Supervision: AV, GSF, ED, MK, RP and PP; Writing—original draft: AV, ED, GSF, RP, MK, PP, IB, LS, AN, MV and EB; Writing—review and editing: all authors.
Funding: This work was supported by WHO through funding from the WHO Solidarity Response Fund and the German Federal Ministry of Health COVID-19 Research and Development.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available on reasonable request. All data can be made available by the authors. Unpublished data supporting the findings of this study are available on the open source Zenodo repository https://zenodo.org/communities/unity-sero-2021?page=1&size=20.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . WHO Coronavirus disease (COVID-19) dashboard. 2021. Available: https://covid19.who.int [PubMed]
- 2.Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome-related Coronavirus 2: A narrative review. Ann Intern Med 2020;172:726–34. 10.7326/M20-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byambasuren O, Cardona M, Bell K, et al. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. J Assoc Med Microbiol Infect Dis Can 2020;5:223–34. 10.3138/jammi-2020-0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh WC, Naing L, Chaw L, et al. What do we know about SARS-Cov-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PLOS ONE 2020;15:e0240205. 10.1371/journal.pone.0240205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and Presymptomatic SARS-Cov-2 infections: A living systematic review and meta-analysis. PLoS Med 2020;17:e1003346. 10.1371/journal.pmed.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanne JH. Covid-19: US cases are greatly underestimated, Seroprevalence studies suggest. BMJ 2020:m2988. 10.1136/bmj.m2988 [DOI] [PubMed] [Google Scholar]
- 7.Wajnberg A, Mansour M, Leven E, et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe 2020;1:e283–9. 10.1016/S2666-5247(20)30120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-Cov-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 9.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021;39:4423–8. 10.1016/j.vaccine.2021.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murhekar MV, Clapham H. COVID-19 Serosurveys for public health decision making. Lancet Glob Health 2021;9:e559–60. 10.1016/S2214-109X(21)00057-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile App for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knabl L, Mitra T, Kimpel J, et al. High SARS-Cov-2 Seroprevalence in children and adults in the Austrian ski resort of Ischgl. Commun Med (Lond) 2021;1:4. 10.1038/s43856-021-00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner A, Guzek A, Ruff J, et al. A longitudinal seroprevalence study in A large cohort of working adults reveals that neutralising SARS-cov-2 RBD-specific antibodies persist for at least six months independent of the severity of symptoms. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2020.12.22.20248604 [DOI]
- 15.Ladage D, Höglinger Y, Ladage D, et al. SARS-Cov-2 antibody prevalence and symptoms in a local Austrian population. Front Med (Lausanne) 2021;8:632942. 10.3389/fmed.2021.632942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boey L, Roelants M, Merckx J, et al. Age-dependent Seroprevalence of SARS-Cov-2 antibodies in school-aged children from areas with low and high community transmission. Eur J Pediatr 2022;181:571–8. 10.1007/s00431-021-04222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokonjic D. A national study of Seroprevalence of COVID-19 infection in the population of the Republika Srpska 2020. WHO unity studies global SARS-Cov-2 Seroepidemiological investigations. data repository for unpublished Datasets for the WHO unity initiative. 4 November, 2021. 2020. Available: https://zenodo.org/communities/unity-sero-2021?page=1&size=20
- 18.Statens Serum Institut . De Første Foreløbige Resultater Af Undersøgelsen for COVID-19 I Befolkningen er NU Klar. 2020. Available: https://www.ssi.dk/aktuelt/nyheder/2020/de-forste-forelobige-resultater-af-undersogelsen-for-covid-19-i-befolkningen-er-nu-klar
- 19.Statens Serum Institute . New preliminary results from the representative Seroprevalence study of COVID-19. 2020. Available: https://files.ssi.dk/notat_foreloebige_resultater_pilotundersoegelse_seropraevalens_COVID-19_29_6_2020
- 20.Statens Serum Institute . COVID-19: the National prevalence survey. results of antibody test with 18,000 invited participants, week 34-36. 2020. Available: https://www.ssi.dk/-/media/arkiv/dk/aktuelt/nyheder/2020/notat---covid-19-prvalensundersgelsen.pdf?la=da
- 21.Petersen MS, Strøm M, Christiansen DH, et al. Seroprevalence of SARS-Cov-2-specific antibodies, Faroe Islands. Emerg Infect Dis 2020;26:2761–3. 10.3201/eid2611.202736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jõgi P, Soeorg H, Ingerainen D, et al. Seroprevalence of SARS-Cov-2 IgG antibodies in two regions of Estonia (Korosero-EST-1). medRxiv 2020;2020. [Google Scholar]
- 23.Koronaepidemian väestöserologiatutkimuksen viikkoraportti . Finnish Institute for health and welfare – THL. 2021. Available: https://www.thl.fi/roko/cov-vaestoserologia/sero_report_weekly.html
- 24.Carrat F, de Lamballerie X, Rahib D, et al. n.d. Seroprevalence of SARS-Cov-2 among adults in three regions of France following the Lockdown and associated risk factors: a Multicohort study. SSRN Journal 10.2139/ssrn.3696820 [DOI]
- 25.Mouravi-Tarkhan O. Sero-Epidemiological cross-sectional investigation on COVID-19 virus infection in Georgia 2020, 2021. WHO unity studies global SARS-Cov-2 Seroepidemiological investigations. data repository for unpublished Datasets for the WHO unity initiative. 2021. Available: https://zenodo.org/communities/unity-sero-2021?page=1&size=20
- 26.Tsertsvadze T, Gatserelia L, Mirziashvili M, et al. SARS-cov-2 antibody seroprevalence in tbilisi, the capital city of country of georgia. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2020.09.18.20195024 [DOI]
- 27.Aziz NA, Corman VM, Echterhoff AKC, et al. Seroprevalence and correlates of SARS-Cov-2 neutralizing antibodies from a population-based study in Bonn, Germany. Nat Commun 2021;12:2117. 10.1038/s41467-021-22351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streeck H, Schulte B, Kümmerer BM, et al. Infection fatality rate of SARS-Cov2 in a super-spreading event in Germany. Nat Commun 2020;11:5829. 10.1038/s41467-020-19509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos-Hövener C, Neuhauser HK, Rosario AS, et al. Serology- and PCR-based cumulative incidence of SARS-Cov-2 infection in adults in a successfully contained early Hotspot (Comolo study), Germany, may to June 2020. Euro Surveill 2020;25:2001752. 10.2807/1560-7917.ES.2020.25.47.2001752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weis S, Scherag A, Baier M, et al. Antibody response using six different serological assays in a completely PCR-tested community after a Coronavirus disease 2019 outbreak—the Conan study. Clin Microbiol Infect 2021;27:470. 10.1016/j.cmi.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkely B, Szabó AJ, Kosztin A, et al. Novel Coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. Geroscience 2020;42:1063–74. 10.1007/s11357-020-00226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagani G, Conti F, Giacomelli A, et al. Seroprevalence of SARS-Cov-2 significantly varies with age: preliminary results from a mass population screening. J Infect 2020;81:e10–2. 10.1016/j.jinf.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanelli P, Bella A, Fedele G, et al. Prevalence of SARS-Cov-2 IgG antibodies in an area of northeastern Italy with a high incidence of COVID-19 cases: a population-based study. Clin Microbiol Infect 2021;27:S1198-743X(20)30709-6. 10.1016/j.cmi.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerriero M, Bisoffi Z, Poli A, et al. Prevalence of asymptomatic SARS-Cov-2-positive individuals in the general population of northern Italy and evaluation of a diagnostic serological ELISA test: a cross-sectional study protocol. BMJ Open 2020;10:e040036. 10.1136/bmjopen-2020-040036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cito F, Amato L, Di Giuseppe A, et al. A COVID-19 Hotspot area: activities and Epidemiological findings. Microorganisms 2020;8:1711. 10.3390/microorganisms8111711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuridin N, Tatyana K. Population-based age-stratified Seroepidemiological investigation protocol for Coronavirus 2019 (COVID-19) infection in Kyrgyz Republic 2021. WHO unity studies global SARS-Cov-2 Seroepidemiological investigations. data repository for unpublished Datasets for the WHO unity initiative. 2021. Available: https://zenodo.org/communities/unity-sero-2021?page=1&size=20
- 37.Snoeck CJ, Vaillant M, Abdelrahman T, et al. Prevalence of SARS-cov-2 infection in the luxembourgish population: the CON-VINCE study. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2020.05.11.20092916 [DOI]
- 38.Vos ERA, den Hartog G, Schepp RM, et al. Nationwide Seroprevalence of SARS-Cov-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. J Epidemiol Community Health 2021;75:489–95. 10.1136/jech-2020-215678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popova AYu, Ezhlova EB, Mel’nikova AA, et al. Experience in studying Seroprevalence to SARS-Cov-2 virus in the population of the Irkutsk region during COVID-19 outbreak. Problemy Osobo Opasnykh Infektsii 2020:106–13. 10.21055/0370-1069-2020-3-106-113 [DOI] [Google Scholar]
- 40.Popova AYu, Ezhlova EB, Mel’nikova AA, et al. Assessment of the herd immunity to SARS-Cov-2 among the population of the Leningrad region during the COVID-19 epidemic. Problemy Osobo Opasnykh Infektsii 2020:114–23. 10.21055/0370-1069-2020-3-114-123 [DOI] [Google Scholar]
- 41.Barchuk A, Skougarevskiy D, Titaev K, et al. Seroprevalence of SARS-Cov-2 antibodies in saint Petersburg, Russia: a population-based study. Sci Rep 2021;11:12930. 10.1038/s41598-021-92206-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popova AYu, Ezhlova EB, Mel’nikova AA, et al. n.d. Herd immunity to SARS-Cov-2 among the population in Saint-Petersburg during the COVID-19 epidemic. Problemy Osobo Opasnykh Infektsii;2020:124–30. 10.21055/0370-1069-2020-3-124-130 [DOI] [Google Scholar]
- 43.Ezhlova P, Yu EBA, Melnikova AA, et al. Distribution of SARS-Cov-2 Seroprevalence among residents of the Tyumen region during the COVID-19 epidemic period. J Microbiol, Epidemiol Immunobiol 2020;97:392–400. [Google Scholar]
- 44.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-Cov-2 in Spain (ENE-COVID): a nationwide, population-based Seroepidemiological study. Lancet 2020;396:535–44. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Public Health Agency of Sweden . Örekomsten AV Antikroppar mot SARS-Cov-2 I Stadsdelen Rinkeby-Kista, Stockholm, 22–24 Juni 2020. report no.: 20129 04/09/2020. 2020. Available: https://www.folkhalsomyndigheten.se/contentassets/2cf102cd299c4382b9a0447dc0626356/forekomsten-antikroppar-rinkeby-kista.pdf
- 46.Roxhed N, Bendes A, Dale M, et al. Multianalyte Serology in home-sampled blood enables an unbiased assessment of the immune response against SARS-Cov-2. Nat Commun 2021;12:3695. 10.1038/s41467-021-23893-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richard A, Wisniak A, Perez-Saez J, et al. Seroprevalence of anti-SARS-cov-2 igg antibodies, risk factors for infection and associated symptoms in geneva, switzerland: a population-based study. Public and Global Health [Preprint]. 10.1101/2020.12.16.20248180 [DOI] [PMC free article] [PubMed]
- 48.Bi Q, Lessler J, Eckerle I, et al. Household transmission of SARS-COV-2: insights from a population-based serological survey. Epidemiology [Preprint] 2020. 10.1101/2020.11.04.20225573 [DOI] [PMC free article] [PubMed]
- 49.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-Cov-2 IgG antibodies in Geneva, Switzerland (Serocov-POP): a population-based study. The Lancet 2020;396:313–9. 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward H, Cooke G, Atchison C, et al. Declining prevalence of antibody positivity to SARS-cov-2: a community study of 365,000 adults. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2020.10.26.20219725 [DOI]
- 51.Wells PM, Doores KJ, Couvreur S, et al. Estimates of the rate of infection and asymptomatic COVID-19 disease in a population sample from SE England. J Infect 2020;81:931–6. 10.1016/j.jinf.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herzog S, De Bie J, Abrams S, et al. Seroprevalence of igg antibodies against SARS coronavirus 2 in belgium – a serial prospective cross-sectional nationwide study of residual samples (march – october 2020). Epidemiology [Preprint]. 10.1101/2020.06.08.20125179 [DOI] [PMC free article] [PubMed]
- 53.Tsaneva-Damyanova D. SARS-Cov-2: Seroepidemiological pattern in northeastern Bulgaria. Biotechnol Biotechnol Equipm 2020;34:441–6. 10.1080/13102818.2020.1772105 [DOI] [Google Scholar]
- 54.Bloomfield M, Pospisilova I, Cabelova T, et al. Searching for COVID-19 antibodies in Czech Children—A needle in the haystack. Front Pediatr 2020;8:597736. 10.3389/fped.2020.597736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenicek K, J., Zrinski Topic R, Stevanovic V, Lukic-Grlic A, Tabain I, Misak Z Seroprevalence of SARS-CoV-2 infection among children in Children’s Hospital Zagreb during the initial and second wave of COVID-19 pandemic in Croatia. (1846-7482 (Electronic)) [DOI] [PMC free article] [PubMed]
- 56.Capai L, Ayhan N, Masse S, et al. Seroprevalence of SARS-Cov-2 IgG antibodies in Corsica (France). J Clin Med 2020;9:3569. 10.3390/jcm9113569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen R, Jung C, Ouldali N, et al. Assessment of spread of SARS-cov-2 by RT-PCR and concomitant serology in children in a region heavily affected by COVID-19 pandemic. Pediatrics [Preprint]. 10.1101/2020.06.12.20129221 [DOI]
- 58.Le Vu S, Jones G, Anna F, et al. Prevalence of SARS-Cov-2 antibodies in France: results from nationwide serological surveillance. Nat Commun 2021;12:3025. 10.1038/s41467-021-23233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bogogiannidou Z, Vontas A, Dadouli K, et al. Repeated Leftover Serosurvey of SARS-Cov-2 IgG antibodies, Greece, March and April 2020. Euro Surveill 2020;25:2001369. 10.2807/1560-7917.ES.2020.25.31.2001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-Cov-2 in Iceland. N Engl J Med 2020;383:1724–34. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sweden PHAo . Påvisning AV Antikroppar Efter Genomgången COVID-19 I Blodprov Från Öppenvården (Delrapport 1). 2020. Available: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/p/pavisning-av-antikroppar-efter-genomgangen-covid-19-i-blodprov-fran-oppenvarden-delrapport-1/
- 62.Public Health Agency of Sweden (Folkhälsomyndigheten) . Påvisning av antikroppar efter genomgången COVID-19 hos blodgivare (Delrapport 2), . 2020Available: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/p/pavisning-av-antikroppar-efter-genomgangen-covid-19-hos-blodgivare-delrapport-2/
- 63.Posfay-Barbe KM, Andrey DO, Virzi J, et al. Prevalence of IgG against SARS-Cov-2 and evaluation of a rapid Medsan IgG test in children seeking medical care. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickson E, Palmateer NE, Murray J, et al. Enhanced surveillance of COVID-19 in Scotland: population-based Seroprevalence surveillance for SARS-Cov-2 during the first wave of the epidemic. Public Health 2021;190:132–4. 10.1016/j.puhe.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Public Health Scotland . Enhanced surveillance of COVID-19 in Scotland: population-based Seroprevalence surveillance. 2020. Available: https://publichealthscotland.scot/publications/enhanced-surveillance-of-covid-19-in-scotland/enhanced-surveillance-of-covid-19-in-scotland-population-based-seroprevalence-surveillance-30-september-2020/ [DOI] [PMC free article] [PubMed]
- 66.Baralija EC, Blazevic M, Cilovic-Lagarija S, et al. Seroepidemiological investigation for coronavirus 2019 (COVID-19) infection in the Federation of Bosnia and Herzegovina. 2020. WHO Unity Studies Global SARS-CoV-2 Seroepidemiological Investigations. Data repository for unpublished datasets for the WHO Unity Initiative, . 2021Available: https://zenodo.org/communities/unity-sero-2021?page=1&size=20
- 67.Pedersen OB, Nissen J, Dinh KM, et al. SARS-Cov-2 infection fatality rate among elderly retired Danish blood donors - A cross-sectional study. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erikstrup C, Hother CE, Pedersen OBV, et al. Estimation of SARS-Cov-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis 2021;72:249–53. 10.1093/cid/ciaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloddonorerne i Danmark . Bloddonorer Testes for Overstået Infektion MED Coronavirus. 2020. Available: www.bloddonor.dk/coronavirus/
- 70.Gallian P, Pastorino B, Morel P, et al. Lower prevalence of antibodies neutralizing SARS-Cov-2 in group O French blood donors. Antiviral Res 2020;181:S0166-3542(20)30294-1. 10.1016/j.antiviral.2020.104880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grzelak L, Temmam S, Planchais C, et al. A comparison of four serological assays for detecting anti-SARS-Cov-2 antibodies in human serum samples from different populations. Sci Transl Med 2020;12:eabc3103. 10.1126/scitranslmed.abc3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer B, Knabbe C, Vollmer T. SARS-Cov-2 IgG Seroprevalence in blood donors located in three different Federal States. Euro Surveill 2020;25:2001285. 10.2807/1560-7917.ES.2020.25.28.2001285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Runkel S, Kowalzik F, Gehring S, et al. Prevalence of severe acute respiratory syndrome Coronavirus-2-specific antibodies in German blood donors during the COVID-19 pandemic. Clin Lab 2020;66:10. 10.7754/Clin.Lab.2020.200915 [DOI] [PubMed] [Google Scholar]
- 74.Percivalle E, Cambiè G, Cassaniti I, et al. Prevalence of SARS-Cov-2 specific Neutralising antibodies in blood donors from the Lodi red zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill 2020;25:24. 10.2807/1560-7917.ES.2020.25.24.2001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiore JR, Centra M, De Carlo A, et al. FAR away from herd immunity to SARS-cov-2: results from a survey in healthy blood donors in south eastern italy. Infectious Diseases (except HIV/AIDS) [Preprint] 2020. 10.1101/2020.06.17.20133678 [DOI] [PMC free article] [PubMed]
- 76.Valenti L, Bergna A, Pelusi S, et al. SARS-cov-2 seroprevalence trends in healthy blood donors during the COVID-19 milan outbreak. Infectious Diseases (except HIV/AIDS) [Preprint]. 10.1101/2020.05.11.20098442 [DOI] [PMC free article] [PubMed]
- 77.Slot E, Hogema BM, Reusken CBEM, et al. Low SARS-Cov-2 Seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat Commun 2020;11:5744. 10.1038/s41467-020-19481-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lundkvist Å, Hanson S, Olsen B. Pronounced difference in COVID-19 antibody prevalence indicates cluster transmission in Stockholm, Sweden. Infect Ecol Epidemiol 2020;10:1806505. 10.1080/20008686.2020.1806505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 22. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/888254/COVID19_Epidemiological_Summary_w22_Final.pdf
- 80.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 39. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/921561/Weekly_COVID19_Surveillance_Report_week_39_FINAL.pdf
- 81.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 38. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/919676/Weekly_COVID19_Surveillance_Report_week_38_FINAL_UPDATED.pdf
- 82.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 37. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/920372/Weekly_COVID19_Surveillance_Report_week_37_FINAL_UPDATED.pdf
- 83.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 36. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/920373/Weekly_COVID19_Surveillance_Report_week_36_UPDATED.pdf
- 84.Public Health England . Weekly Coronavirus disease 2019 (COVID-19) surveillance report: week 35. 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/912973/Weekly_COVID19_Surveillance_Report_week_35_FINAL.PDF
- 85.Thompson CP, Grayson NE, Paton RS, et al. Detection of Neutralising antibodies to SARS-Cov-2 to determine population exposure in Scottish blood donors between March and may 2020. Euro Surveill 2020;25:2000685. 10.2807/1560-7917.ES.2020.25.42.2000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fogel O, Mariaggi AA, Méritet JF, et al. Étude prospective de Séroprévalence Du Sars-Cov-2 Chez 249 patients Suivis pour UN Rhumatisme Inflammatoire Chronique. Revue Du Rhumatisme 2020;87:A292–3. 10.1016/j.rhum.2020.10.531 [DOI] [Google Scholar]
- 87.Choi M, Bachmann F, Naik MG, et al. Low Seroprevalence of SARS-Cov-2 antibodies during systematic antibody screening and serum responses in patients after COVID-19 in a German transplant center. J Clin Med 2020;9:11.:3401. 10.3390/jcm9113401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rauber C, Tiwari-Heckler S, Pfeiffenberger J, et al. SARS-cov-2 seroprevalence and clinical features of COVID-19 in a german liver transplant recipient cohort: a prospective serosurvey study. Transplantation [Preprint] 2020. 10.1101/2020.09.30.20204537 [DOI] [PMC free article] [PubMed]
- 89.Zambelli A, Fotia V, Bosetti T, et al. Prevalence and clinical impact of asymptomatic or mildly symptomatic Sarscov-2 infection among actively treated cancer patients during COVID-19 pandemic in Italy. Ann Oncol 2020;31:S994. 10.1016/j.annonc.2020.08.1741 [DOI] [Google Scholar]
- 90.Medas F, Cappellacci F, Anedda G, et al. Seroprevalence of SARS-Cov-2 in the setting of a non-dedicated COVID-19 hospital in a low Cov-2 incidence area: implications for surgery. Ann Med Surg (Lond) 2020;60:261–2. 10.1016/j.amsu.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Capasso N, Palladino R, Montella E, et al. Prevalence of SARS-Cov-2 antibodies in multiple sclerosis: the hidden part of the iceberg. J Clin Med 2020;9:12.:4066. 10.3390/jcm9124066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cento V, Alteri C, Merli M, et al. Effectiveness of infection-containment measures on SARS-Cov-2 Seroprevalence and circulation from May to. PLoS One 2020;15:e0242765. 10.1371/journal.pone.0242765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berte’ R, Mazza S, Stefanucci MR, et al. Seroprevalence of SARS-Cov2 in IBD patients treated with biologic therapy. J Crohns Colitis 2021;15:864–8. 10.1093/ecco-jcc/jjaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vena A, Berruti M, Adessi A, et al. Prevalence of antibodies to SARS-Cov-2 in Italian adults and associated risk factors. JCM 2020;9:2780. 10.3390/jcm9092780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, et al. Seroprevalence of SARS-Cov-2-specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data. Cancer Treat Rev 2020;90:S0305-7372(20)30140-7. 10.1016/j.ctrv.2020.102102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prados N, González-Ravina C, Vergara V, et al. Risk factor analysis for Sars-Cov-2 Seropositivity within assisted reproductive. Fertility and Sterility 2020;114:e547. 10.1016/j.fertnstert.2020.09.077 [DOI] [Google Scholar]
- 97.Clarke C, Prendecki M, Dhutia A, et al. High prevalence of asymptomatic COVID-19 infection in Hemodialysis patients detected using serologic screening. JASN 2020;31:1969–75. 10.1681/ASN.2020060827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prendecki M, Clarke C, Gleeson S, et al. Detection of SARS-Cov-2 antibodies in kidney transplant recipients. J Am Soc Nephrol 2020;31:2753–6. 10.1681/ASN.2020081152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Egerup P, Fich Olsen L, Christiansen A-MH, et al. Severe acute respiratory syndrome Coronavirus 2 (SARS-Cov-2) antibodies at delivery in women, partners, and newborns. Obstetrics & Gynecology 2021;137:49–55. 10.1097/AOG.0000000000004199 [DOI] [PubMed] [Google Scholar]
- 100.Mattern J, Vauloup-Fellous C, Zakaria H, et al. Post Lockdown COVID-19 Seroprevalence and circulation at the time of delivery, France. PLoS One 2020;15:e0240782. 10.1371/journal.pone.0240782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsatsaris V, Mariaggi A-A, Launay O, et al. SARS-COV-2 IgG antibody response in pregnant women at delivery. J Gynecol Obstet Hum Reprod 2021;50:102041. 10.1016/j.jogoh.2020.102041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cosma S, Borella F, Carosso A, et al. “The “scar” of a pandemic: cumulative incidence of COVID‐19 during the first trimester of pregnancy”. J Med Virol 2021;93:537–40. 10.1002/jmv.26267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crovetto F, Crispi F, Llurba E, et al. SEROPREVALENCE and clinical spectrum of sars-cov-2 infection in the first versus third trimester of pregnancy. Obstetrics and Gynecology [Preprint]. 10.1101/2020.06.17.20134098 [DOI]
- 104.Villalaín C, Herraiz I, Luczkowiak J, et al. Seroprevalence analysis of SARS-Cov-2 in pregnant women along the first pandemic outbreak and perinatal outcome. PLoS One 2020;15:e0243029. 10.1371/journal.pone.0243029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lumley SF, Eyre DW, McNaughton AL, et al. SARS-Cov-2 antibody prevalence, Titres and Neutralising activity in an Antenatal cohort. Eurosurveillance 2020;25. 10.2807/1560-7917.ES.2020.25.41.2001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krátká Z Fau - Fürst T, Fürst T Fau - Vencálek O, Vencálek O Fau - Kůrková V, et al. n.d. Exploratory drilling: how to set up, carry out, and evaluate a Seroprevalence study. (0008-7335 (print)). [PubMed]
- 107.Jerković I, Ljubić T, Bašić Ž, et al. SARS-Cov-2 antibody Seroprevalence in industry workers in split-Dalmatia and Šibenik-Knin County, Croatia. J Occup Environ Med 2021;63:32–7. 10.1097/JOM.0000000000002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vince A, Zadro R, Šostar Z, et al. SARS-cov-2 seroprevalence in a cohort of asymptomatic, RT-PCR negative croatian first league football players. Sports Medicine [Preprint] 2020. 10.1101/2020.10.30.20223230 [DOI]
- 109.Anna F, Goyard S, Lalanne AI, et al. High Seroprevalence but short-lived immune response to SARS-Cov-2 infection in Paris. Eur J Immunol 2021;51:180–90. 10.1002/eji.202049058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fontanet A, Tondeur L, Grant R, et al. SARS-Cov-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission. Euro Surveill 2021;26:2001695. 10.2807/1560-7917.ES.2021.26.15.2001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roederer T, Mollo B, Vincent C, et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health 2021;6:e202–9. 10.1016/S2468-2667(21)00001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krähling V, Kern M, Halwe S, et al. Epidemiological study to detect active SARS-Cov-2 infections and Seropositive persons in a selected cohort of employees in the Frankfurt am main metropolitan area. Epidemiology [Preprint]. 10.1101/2020.05.20.20107730 [DOI]
- 113.Mack D, Gärtner BC, Rössler A, et al. Prevalence of SARS-Cov-2 IgG antibodies in a large prospective cohort study of elite football players in Germany (may-June 2020): implications for a testing protocol in asymptomatic individuals and estimation of the rate of undetected cases. Clin Microbiol Infect 2021;27:473.:S1198-743X(20)30729-1. 10.1016/j.cmi.2020.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ceban A. National agency for public health Rom. serological surveillance of COVID-19 in the general population with age stratification in the Republic of Moldova 2020. WHO unity studies global SARS-Cov-2 Seroepidemiological investigations. data repository for unpublished Datasets for the WHO unity initiative. November 4, 2021. 2021. Available: https://zenodo.org/communities/unity-sero-2021?page=1&size=20
- 115.Gujski M, Jankowski M, Pinkas J, et al. Prevalence of current and past SARS-Cov-2 infections among police employees in Poland, June-July 2020. JCM 2020;9:3245. 10.3390/jcm9103245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pérez-García F, Pérez-Zapata A, Arcos N, et al. Severe acute respiratory Coronavirus virus 2 (SARS-Cov-2) infection among hospital workers in a severely affected institution in Madrid, Spain: A surveillance cross-sectional study. Infect Control Hosp Epidemiol 2021;42:803–9. 10.1017/ice.2020.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ulyte A, Radtke T, Abela IA, et al. Clustering and longitudinal change in SARS-Cov-2 Seroprevalence in school children in the Canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ 2021;372:n616. 10.1136/bmj.n616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roarty C, Tonry C, McFetridge L, et al. Kinetics and Seroprevalence of SARS-Cov-2 antibodies in children. Lancet Infect Dis 2021;21:S1473-3099(20)30884-7. 10.1016/S1473-3099(20)30884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waterfield T, Watson C, Moore R, et al. Seroprevalence of SARS-Cov-2 antibodies in children: a prospective Multicentre cohort study. Arch Dis Child 2021;106:680–6. 10.1136/archdischild-2020-320558 [DOI] [PubMed] [Google Scholar]
- 120.Armann JP, Unrath M, Kirsten C, et al. n.d. Anti-SARS-Cov-2 IgG antibodies in adolescent students and their teachers in Saxony, Germany (Schoolcovidd19): very low Seropraevalence and transmission rates. SSRN Journal 10.2139/ssrn.3651210 [DOI] [Google Scholar]
- 121.Reisinger EC, von Possel R, Warnke P, et al. Mütter-screening in Einem COVID-19-Niedrig-Pandemiegebiet: Bestimmung SARS-Cov-2-Spezifischer Antikörper BEI 401 Rostocker Müttern Mittels ELISA und Immunfluoreszenz-Bestätigungstest. Dtsch Med Wochenschr 2020;145:e96–100. 10.1055/a-1197-4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsitsilonis OE, Paraskevis D, Lianidou E, et al. Seroprevalence of antibodies against SARS-Cov-2 among the personnel and students of the national and Kapodistrian University of Athens, Greece: A preliminary report. Life (Basel) 2020;10:214. 10.3390/life10090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lastrucci V, Lorini C, Del Riccio M, et al. SARS-Cov-2 Seroprevalence survey in people involved in different essential activities during the general lock-down phase in the province of Prato (Tuscany, Italy). Vaccines (Basel) 2020;8:778. 10.3390/vaccines8040778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soriano V, Meiriño R, Corral O, et al. Severe acute respiratory syndrome Coronavirus 2 antibodies in adults in Madrid, Spain. Clin Infect Dis 2021;72:1101–2. 10.1093/cid/ciaa769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montenegro P, Brotons C, Serrano J, et al. Community Seroprevalence of COVID-19 in probable and possible cases at primary health care centres in Spain. Fam Pract 2021;38:154–9. 10.1093/fampra/cmaa096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Emmenegger M, Cecco E, Lamparter D, et al. n.d. Early peak and rapid decline of SARS-Cov-2 Seroprevalence in a Swiss metropolitan region. medRxiv;2020:2020. [Google Scholar]
- 127.Castro Dopico X, Muschiol S, Christian M, et al. Seropositivity in blood donors and pregnant women during the first year of SARS-Cov-2 transmission in Stockholm, Sweden. J Intern Med 2021;290:666–76. 10.1111/joim.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davis KAS, Carr E, Leightley D, et al. Indicators of past COVID-19 infection status: findings from a large occupational cohort of staff and postgraduate research students from a UK university. Epidemiology [Preprint]. 10.1101/2020.12.07.20245183 [DOI] [PMC free article] [PubMed]
- 129.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational Epidemiological studies reporting prevalence and cumulative incidence data. JBI Evidence Implementation 2015;13:147–53. [DOI] [PubMed] [Google Scholar]
- 130.Van Walle I, Leitmeyer K, Broberg EK. Meta-analysis of the clinical performance of commercial SARS-cov-2 nucleic acid, antigen and antibody tests up to 22 august 2020. Public and Global Health [Preprint] 2020. 10.1101/2020.09.16.20195917 [DOI] [PMC free article] [PubMed]
- 131.Bergeri I, Lewis HC, Subissi L, et al. Early Epidemiological investigations: world health organization UNITY protocols provide a standardized and timely International investigation framework during the COVID-19 pandemic. Influenza Other Respir Viruses 2022;16:7–13. 10.1111/irv.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jõgi P, Soeorg H, Ingerainen D. Seroprevalence of SARS-Cov-2 IgG antibodies in two regions of Estonia (Korosero-Est-1). [Preprint] 2020. 10.1101/2020.10.21.20216820 [DOI]
- 133.Bobrovitz N, Arora RK, Cao C, et al. Global Seroprevalence of SARS-Cov-2 antibodies: A systematic review and meta-analysis. PLOS ONE 2021;16:e0252617. 10.1371/journal.pone.0252617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-Cov-2 Seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 2021;27:331–40. 10.1016/j.cmi.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-Cov-2: a systematic review and meta-analysis. Lancet Glob Health 2021;9:e598–609. 10.1016/S2214-109X(21)00026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bergeri I, Whelan MG, Ware H, et al. Global SARS-Cov-2 Seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLOS Med 2022;19:e1004107. 10.1371/journal.pmed.1004107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bobrovitz N, Arora RK, Cao C, et al. Global Seroprevalence of SARS-Cov-2 antibodies: A systematic review and meta-analysis. PLoS One 2021;16:e0252617. 10.1371/journal.pone.0252617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaselli NM, Hungerford D, Shenton B, et al. The seroprevalence of SARS-cov-2 in europe: A systematic review. Microbiology [Preprint]. 10.1101/2021.04.12.439425 [DOI] [PMC free article] [PubMed]
- 139.Goldman M, Steele WR, Di Angelantonio E, et al. Comparison of donor and general population demographics over time: a BEST collaborative group study. Transfusion 2017;57:2469–76. 10.1111/trf.14307 [DOI] [PubMed] [Google Scholar]
- 140.Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS‐Cov‐2. Cochrane Database Syst Rev 2020;6:CD013652. 10.1002/14651858.CD013652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for COVID-19: systematic review and meta-analysis. BMJ 2020;370:m2516. 10.1136/bmj.m2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.World Health Organization . Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody, . 2020Available: https://www.who.int/publications/m/item/WHO-BS-2020.2403
- 143.Kristiansen PA, Page M, Bernasconi V, et al. WHO International standard for anti-SARS-Cov-2 immunoglobulin. Lancet 2021;397:1347–8. 10.1016/S0140-6736(21)00527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.The JRC releases new reference materials for the quality control of SARS-Cov-2 antibody tests. European Centre for Disease Prevention and Control 2021. Available: https://ec.europa.eu/jrc/en/news/new-reference-materials-quality-control-covid-19-antibody-tests [Google Scholar]
- 145.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-Cov-2 infection in humans. Nat Microbiol 2020;5:1598–607. 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-Cov-2 Neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2021;2:e240–9. 10.1016/S2666-5247(21)00025-2 Available: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00025-2/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clapham H Fau - Hay J, Hay J Fau - Routledge I, Routledge I Fau - Takahashi S, et al. n.d. Seroepidemiologic study designs for determining SARS-COV-2 transmission and immunity. (1080-6059 (electronic)). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064240supp001.pdf (120.7KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available on reasonable request. All data can be made available by the authors. Unpublished data supporting the findings of this study are available on the open source Zenodo repository https://zenodo.org/communities/unity-sero-2021?page=1&size=20.