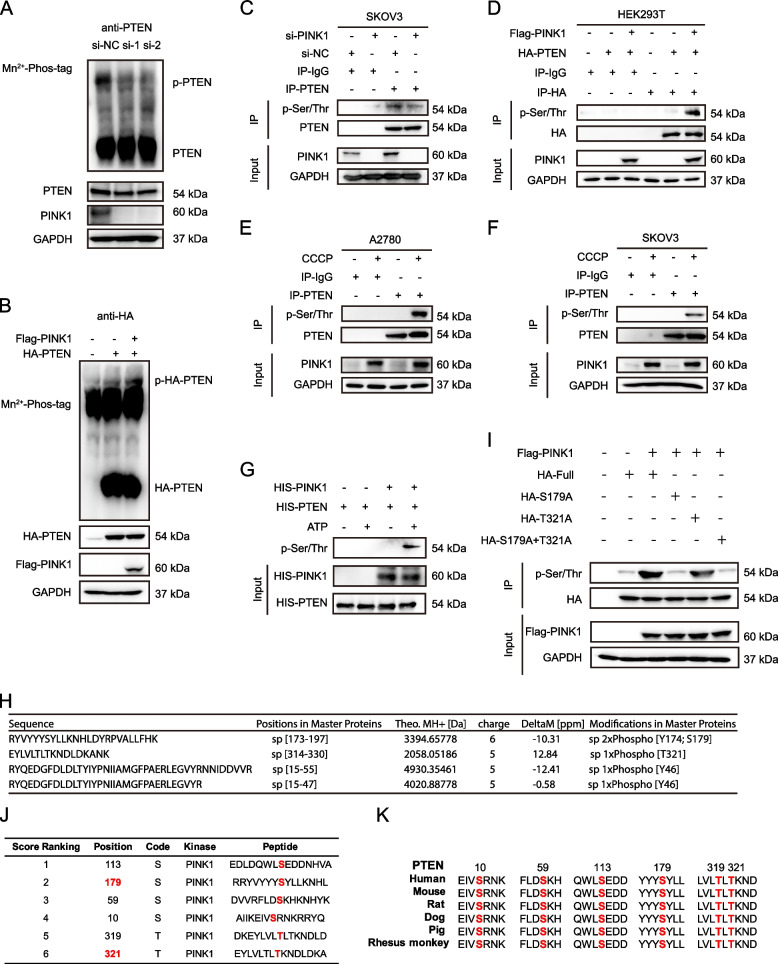
Fig. 4.
PINK1 phosphorylates PTEN at residue Ser179. A Phosphorylation of endogenous PTEN was detected through phos-tag gels upon the knockdown of PINK1 in SKOV3 cells. The lysates were subjected to SDS-PAGE containing Mn2+–Phos-tag, followed by immunoblotting with the anti-PTEN antibody. B Phosphorylation of exogenous PTEN was detected through phos-tag gels with or without the over-expression of Flag-PINK1 and HA-PTEN in HEK293T cells. The lysates were treated as above and followed by immunoblotting with the anti-HA antibody. C PTEN was immunopurified from SKOV3 cells with or without PINK1 knockdown. The phosphorylation on serine/threonine of PTEN was detected by immunoblotting with the anti-pan Phospho-Serine/Threonine (pan p-Ser/Thr) primary antibody. D PTEN was immunopurified from HEK293T cells with or without over-expression of Flag-PINK1 and HA-PTEN. The Phosphorylation on serine/threonine of PTEN protein was detected by immunoblotting with the anti-pan p-Ser/Thr primary antibody. E and F PTEN was immunopurified from A2780 (E) or SKOV3 (F) cells with or without treatment of 10μM CCCP for 6 h. The phosphorylation on serine/threonine of PTEN protein was detected by immunoblotting with the anti-pan p-Ser/Thr primary antibody. G In vitro kinase assay. The active kinase, PINK1, and the substrate PTEN, in the presence or absence of adenosine 5’-triphosphate (ATP), were used for kinase assay. Anti-pan p-Ser/Thr primary antibody was applied for detecting phospho-serine/threonine. H Phosphorylated residues in PTEN detected by mass spectrometry analysis. I Phosphorylation on serine/threonine of PTEN protein immunopurified from HEK293T cells with PTEN mutant over-expression was detected through western blot, immunoblotting with the anti-pan p-Ser/Thr antibody. J Phosphorylation sites predicted by GPS 6.0. K Sequence conservation analysis of relevant amino acids of PTEN