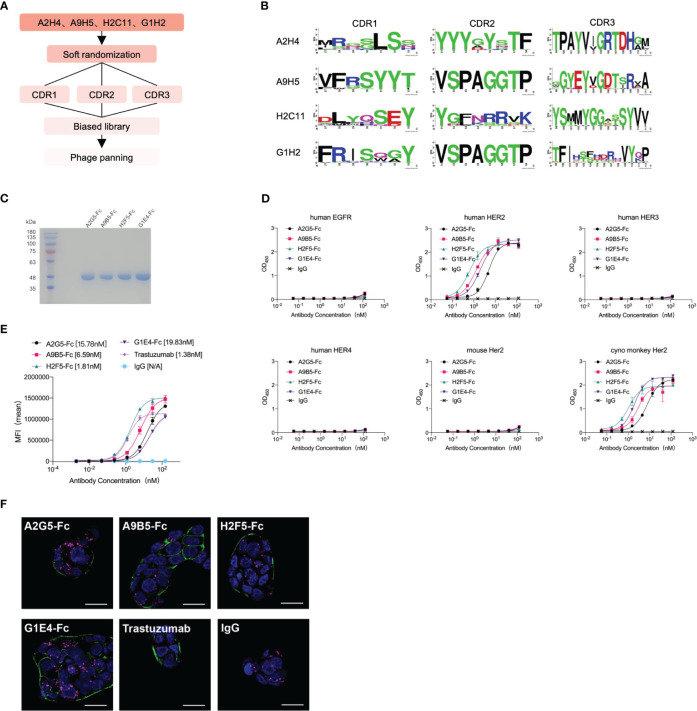
Figure 2.
Affinity maturation of HER2-targeted nanobodies by phage display and identification of VHH-Fc. (A) The flowchart summarized the overall process of affinity maturation. (B) The sequence alignments of CDR regions were represented by Weblogo (http://weblogo.berkeley.edu/logo.cgi). All CDR regions of A2H4 and H2C11 were designed to generate random mutations. As for A9H5 and G1H2, their CDR2 region remained unchanged. (C) VHH-Fc fusions were purified from the 293T system and resolved by SDS-PAGE. (D) VHH-Fc derived from HER2-nanobodies with high affinity retained target specificity and species cross-reactivity. VHH-Fc showed a potent binding activity to human HER2 and monkey Her2 recombinant proteins. (E) VHH-Fc exhibited greater saturation activity in tumor cells compared to related parental nanobodies. (F) Confocal images of NCI-N87 cells identified the binding of VHH-Fc to the cell surface. Cells were treated with 50 nM antibodies and were stained by DyLight® 488 for detection of the HER2-antibody complex. Alexa-Fluor 647-labeled secondary antibody and DAPI were used to visualize lysosomes and nuclei, respectively. Scale bars = 10 μm.