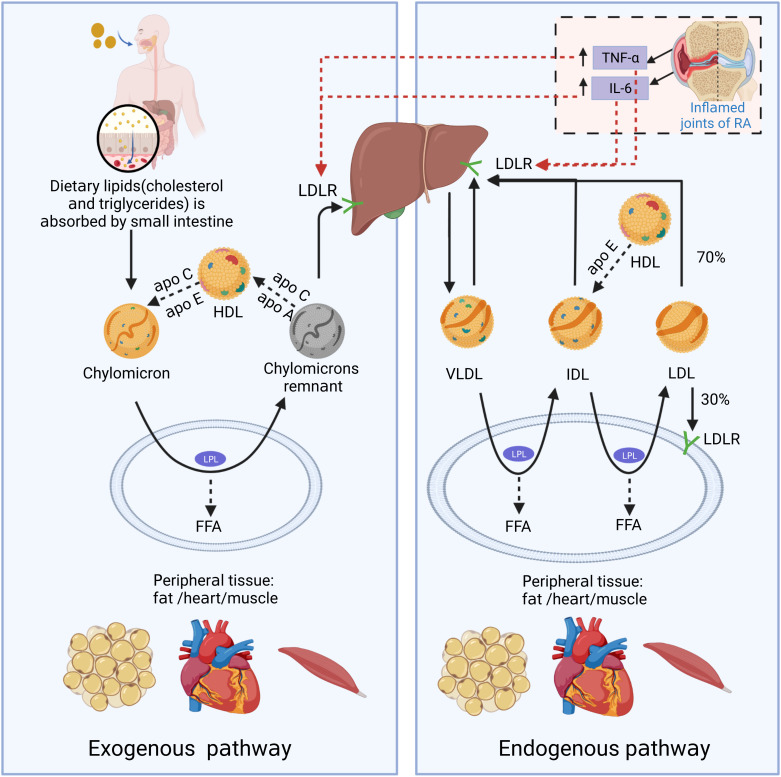
Figure 2.
The schematic diagram of endogenous and exogenous cholesterol pathways. In the exogenous regulation pathway, lipids (cholesterol and triglycerides) from food sources are transferred to CMs through being absorbed into the blood in the small intestine. CMs reach peripheral tissues with the blood and exchange lipoproteins with HDL to obtain apolipoprotein E (Apo E). And CMs is metabolized by lipoprotein lipase (LPL) in fat and muscle cells to generate free fatty acids (FFAs) and chylomicron remnants. FFAs are absorbed by adjacent muscle and fat cells for energy production or storage. As the size of chylomicron decreases, the phospholipids and carrier proteins (Apo A and C) on the surface of the chylomicron are transferred to other lipoproteins (primarily HDL). Apo E on chylomicron remnants binds to LDL receptor (LDLR) and other liver receptors (such as low-density lipoprotein receptor-related protein 1 and syndecan-4), and is absorbed and cleared by liver cells. In the endogenous lipoprotein pathway, VLDL is produced by the liver, and enters the blood. TGs in VLDL is metabolized by LPL in peripheral tissues and generate FFAs and IDL. Those IDL particles are relatively enriched in cholesterol esters and obtain Apo E from HDL particles. In a pathway similar to the removal of chylomicron remnants, a small portion (about 50%) of the IDL particles can be removed from circulation by the liver through binding with Apo E and liver receptors, such as LDL and LRP receptors. The remaining TGs in IDL continue to release FFAs by LPL action, and the exchangeable carrier proteins are transferred from IDL particles to other lipoproteins, generating low-density lipoprotein (LDL). About 70% of circulating LDL is cleared by liver cell LDLR-mediated endocytosis, and the rest is absorbed by extra-liver tissues. In the state of RA inflammation, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) produced locally in the joints can enter the circulation. TNF-α and IL-6 can promote LDL metabolism by increasing the expression of LDLR on the surface of liver cells.