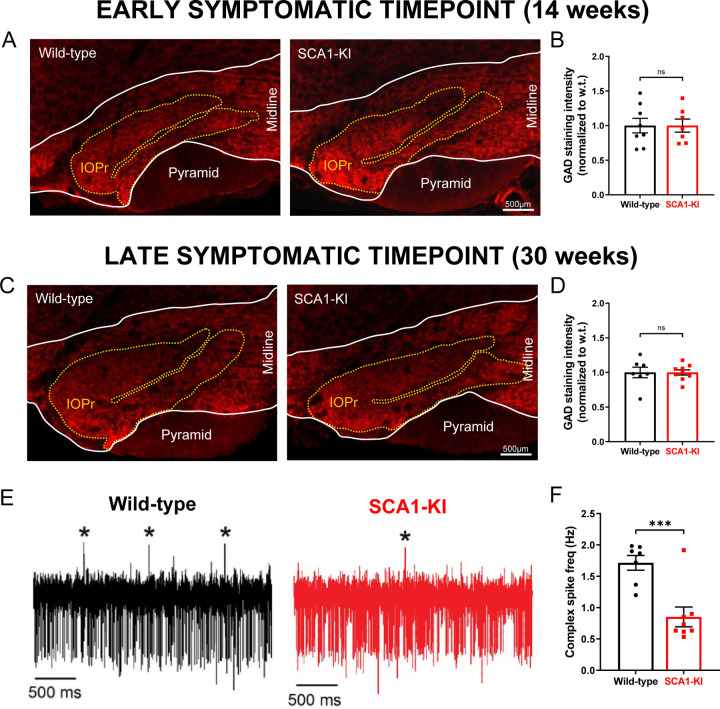
Figure 3. Inhibitory synaptic inputs to the IO remain intact in SCA1-KI mice.
(A) Coronal histological sections showing the IOPr in wild-type (left) and SCA1-KI (right) mice at an early symptomatic timepoint of 14 weeks. Sections have been stained for glutamate decarboxylase (GAD), a marker of GABAergic terminals (red). (B) Quantification of staining reveals that GAD signal is retained in the SCA1-KI IO at 14 weeks. (C) Coronal histological sections showing GAD staining in the principal IO at a late symptomatic timepoint of 30 weeks. (D) Quantification of staining reveals that GAD signal is also retained in the SCA1-KI IO at 30 weeks. (E) Representative traces of in-vivo cerebellar spiking patterns in head-fixed, awake mice at 14 weeks. Complex spikes (generated by IO neurons) are indicated with asterisks (*). (F) Complex spike frequency is reduced in SCA1-KI cerebella, suggesting that IO neurons in-vivo are not disinhibited by loss of inhibitory synaptic input. Data are expressed as mean ± SEM. Statistical significance derived by unpaired t-test with Welch’s correction, *** = P < 0.001, ns = not significant. (A–D) 14 weeks: Nwild-type = 8 mice, NSCA1-KI = 7 mice; 30 weeks: Nwild-type = 7 mice, NSCA1-KI = 9 mice. (E,F) Nwild-type = 7 mice, NSCA1-KI = 8 mice.