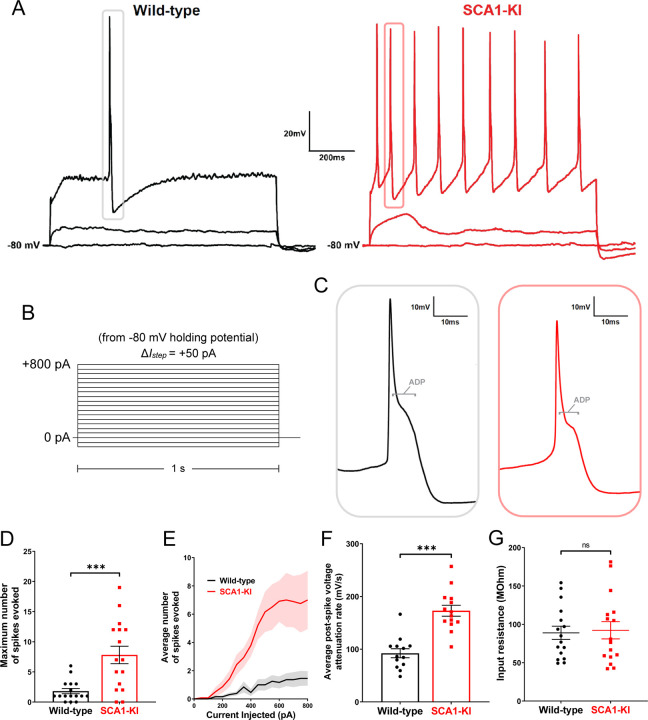
Figure 6. IOPr neurons in SCA1-KI mice are hyperexcitable.
(A) Representative traces of evoked activity in IO neurons from wild-type (left) and SCA1-KI (right) mice at an early symptomatic timepoint (14wks). Traces shown for each group are 0 pA injected (bottom trace), +200 pA injected (middle trace), and +550 pA injected (top trace). (B) From a holding potential of −80 mV, a range of 0–800 pA depolarizing currents were injected in +50 pA, 1 second steps. Recordings were performed in current clamp mode. (C) Inset of representative evoked spikes at a higher timescale resolution reveal a long afterdepolarization (ADP) “hump,” a characteristic feature of IOPr neurons. (D) Unlike IO neurons in wild-type mice, IO neurons in SCA1-KI mice are able to sustain a spike train. (E) Input-output curve of average spikes produced in IO neurons of each genotype. Number of spikes rose steadily with current injection in SCA1-KI IO neurons, while wild-type IO neurons rarely exhibited spiking in the range depicted (+0−800 pA injected). (F) The rate at which membrane potential recovered back to baseline from its minimum value post-spike was significantly higher in SCA1-KI IOPr neurons, allowing these cells to fire repetitively. (G) Input resistance of IO neurons is unchanged in SCA1-KI mice, demonstrating that this hyperexcitability phenotype is not a product of any change in voltage generated per injected current step. Data are expressed as mean ± SEM. Statistical significance derived by unpaired t-test with Welch’s correction, *** = P < 0.001, ns = not significant. (D,E,G) Nwild-type = 16 cells from 14 mice, NSCA1-KI = 16 cells from 11 mice (F) Non-firing cells removed, so Nwild-type = 13 cells from 12 mice, NSCA1-KI = 14 cells from 9 mice.