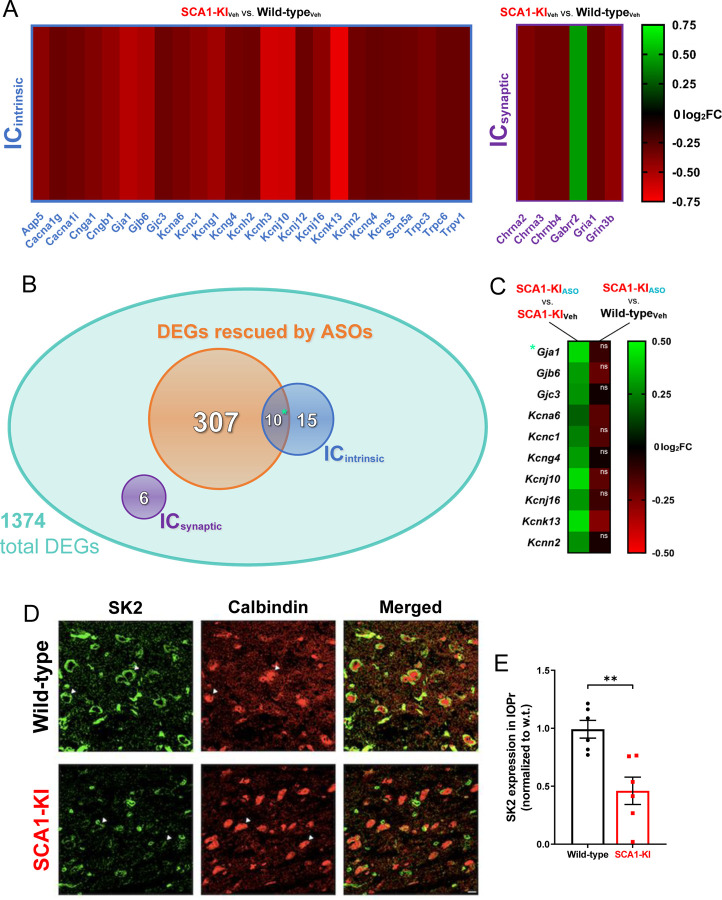
Figure 7. Ion channel expression is disrupted in the SCA1-KI medulla.
(A) Transcriptomic analysis of whole medulla from SCA1-KI and wild-type mice at 28 weeks reveal that 31 ion channel genes are differentially expressed. Of these, 25 influence excitability intrinsically (ICintrinsic), while 6 influence excitability synaptically (ICsynaptic). (B) Downregulation of Atxn1 expression by antisense oligonucleotides (ASOs) ameliorates brainstem phenotypes in SCA1-KI mice. Of the 1374 differentially expressed genes (DEGs) in the SCA1-KI medulla, 317 DEGs were significantly rescued by ASO treatment (and, therefore, are more likely to be responsible for the observed phenotypic rescue). Within this cohort, ICintrinsic genes were significantly enriched. (C) Heatmap showing differential expression of the 10 ICintrinsic DEGs between treatment groups. These ion channel genes exhibited a significant increase in expression after ASO treatment (left), with the majority of them rising to wild-type levels (right). (D) In order to examine how decreases in medullary ion channel transcripts may result in a loss of channels in the IOPr, immunostaining for the small-conductance potassium channel SK2 was performed. Coronal histological sections of the IOPr in wild-type (top) and SCA1-KI (bottom) mice at 14 weeks (an early symptomatic timepoint) are shown. Sections have been stained for SK2 (green) and calbindin (red). (E) Quantification of immunostaining reveals a significant loss of SK2 channels on the membrane of SCA1-KI IOPr neurons. Data are expressed as mean ± SEM. Statistical significance derived by Fischer’s exact test (enrichment analyses) or unpaired t-test with Welch’s correction (all other comparisons), ** = P < 0.01, ns = not significant. (A–C) Nwild-type, Vehicle = 8 mice, NSCA1-KI, Vehicle = 8 mice, NSCA1-KI, ASO = 7 mice. (D, E) Nwild-type = 6 mice, NSCA1-KI = 6 mice.