Keywords: immunity, macrophages, monocytes, pathogenesis, pulmonary fibrosis
Abstract
Pulmonary fibrosis results from a plethora of abnormal pathogenetic events. In idiopathic pulmonary fibrosis (IPF), inhalational, environmental, or occupational exposures in genetically and epigenetically predisposed individuals trigger recurrent cycles of alveolar epithelial cell injury, activation of coagulation pathways, chemoattraction, and differentiation of monocytes into monocyte-derived alveolar macrophages (Mo-AMs). When these events happen intermittently and repeatedly throughout the individual’s life cycle, the wound repair process becomes aberrant leading to bronchiolization of distal air spaces, fibroblast accumulation, extracellular matrix deposition, and loss of the alveolar-capillary architecture. The role of immune dysregulation in IPF pathogenesis and progression has been underscored in the past mainly after the disappointing results of immunosuppressant use in IPF patients; however, recent reports highlighting the prognostic and mechanistic roles of monocytes and Mo-AMs revived the interest in immune dysregulation in IPF. In this review, we will discuss the role of these cells in the onset and progression of IPF, as well as potential targeted therapies.
INTRODUCTION
Pulmonary fibrosis (PF) represents the end stage of several, heterogeneous interstitial lung diseases (1, 2). More than 200 causes of PF have been described including autoimmune diseases, genetic disorders, drugs, and environmental and occupational exposure to injurious stimuli. If none of these causes is identified, patients with usual interstitial pneumonia pattern are diagnosed with idiopathic pulmonary fibrosis (IPF) (3).
A plethora of abnormal pathogenetic events happening intermittently and repeatedly over time lead to IPF development in genetically and epigenetically predisposed individuals of advanced age. The disease initiates with alveolar epithelial cell injury, activation of coagulation pathways and chemoattraction, and differentiation of monocytes into monocyte-derived alveolar macrophages (Mo-AMs). When these events become cyclical and recur over time, key cells participating in this process become senescent and develop abnormal metabolism and processes leading to aberrant wound repair, characterized by fibroblast accumulation, extracellular matrix deposition, bronchiolization of distal air spaces, and loss of the alveolar-capillary architecture (4–21). As the disease progresses, the immune system becomes dysregulated, and exaggerated immune responses lead to increased IPF mortality (22–24). Recent reports demonstrated that increased monocyte counts and monocyte-specific genes as well as decreased T-cell subpopulations and T-cell costimulatory pathways have been associated with increased risk of IPF mortality and poor disease outcomes (23–29). The aforementioned, coupled with experimental evidence showing that newly recruited monocyte-derived macrophages contribute to lung fibrosis pathogenesis and progression after alveolar epithelium injury through the secretion of several profibrotic mediators, led to a revived interest in the role of immune dysregulation in IPF and highlights the importance of monocytes and macrophages as key immune cells participating in the process of aberrant lung injury and repair (30–32). In this review, we will discuss the emerging role of monocytes and macrophages in PF pathogenesis and progression and highlight mechanistic insights.
POPULATIONS OF MACROPHAGES IN THE HEALTHY LUNG
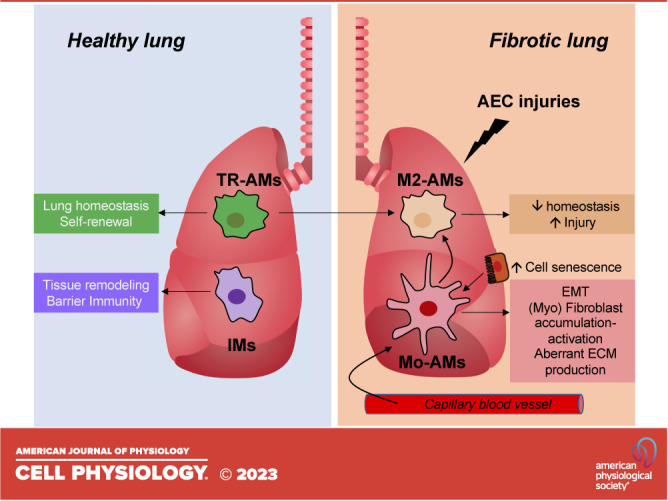
Macrophages, the most abundant immune cell type in healthy lungs, can be classified into two main populations: alveolar macrophages (AMs) and interstitial macrophages (IMs). AMs reside in the lumen of distal lung alveoli as well as in the interalveolar septum in proximity to pneumocytes. AMs are the primary resident innate immune cells in the lungs and exert a critical role in tissue homeostasis: they recognize and clear inhaled pathogens and debris, catabolize surfactants, and orchestrate the initiation and resolution of inflammation (33). Like other tissue-resident macrophages, AMs represent a self-renewing population that originates from embryonic progenitors and requires minimal input from peripheral blood monocytes in normal conditions (34–36). Following lung injury, circulating monocytes extravasate from blood vessels and infiltrate the tissue where they differentiate into Mo-AMs, further enriching the pool of resident AMs (37) (Fig. 1).
Figure 1.
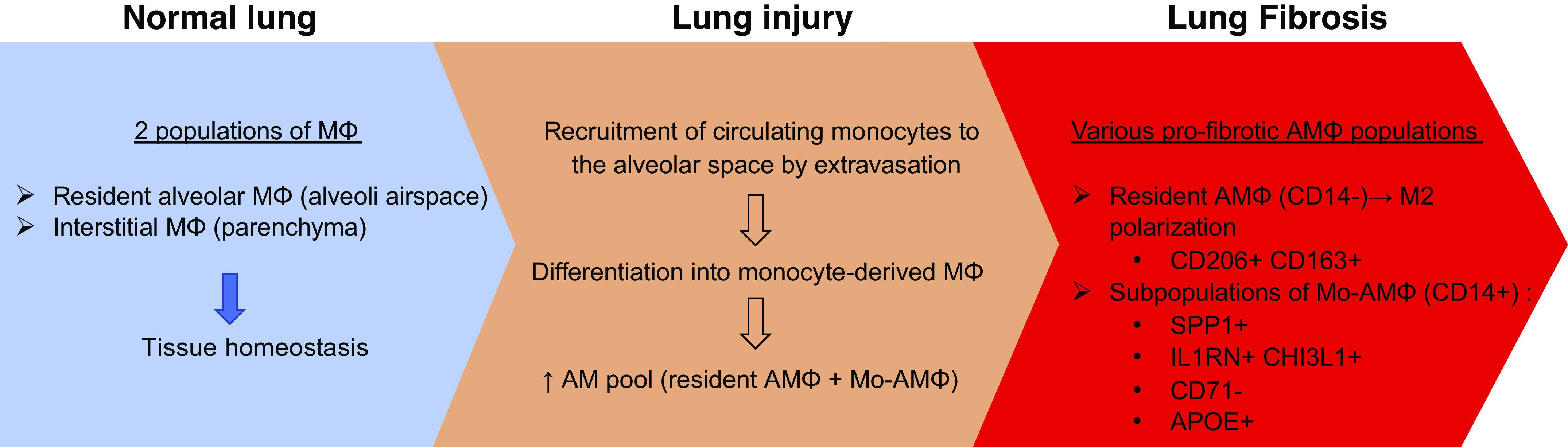
Emergence of monocyte (M) and alveolar macrophage (AM) populations in pulmonary fibrosis. Mo-AM, monocyte-derived alveolar macrophage.
Interstitial macrophages (IMs) are located in the lung interstitium. Compared to AMs, IMs are smaller, with a size and morphology similar to blood monocytes. Little is known about the potential proinflammatory activities of IMs, and most of the studies have focused on their potential immunoregulatory properties. Indeed, mouse and human IMs have been shown to express the immunosuppressive cytokine IL-10 in the steady state (38). IMs are also involved in tissue remodeling and participate in barrier immunity as antigen-presenting cells (33). An additional population of macrophages called intravascular macrophages, attached to the capillary endothelium, has also been described in humans and other mammals. However, their role remains poorly documented (33, 39).
THE MACROPHAGE POLARIZATION PARADIGM
Macrophages are cells with a high degree of plasticity and display a remarkable capacity to switch from one phenotype to another (40). Beyond the AM/IM macrophage subtypes, polarized activation of macrophages distinguishes classically activated (M1) and alternatively activated (M2) macrophages, the latter being divided into M2a, M2b, M2c, and M2d subcategories (40). The central suggested concept is that M1 macrophages suppress, while M2 macrophages promote, fibrosis and aberrant wound healing (31).
M1 macrophage polarization occurs upon exposure to inflammatory molecules [e.g., T helper (Th)1 cell-secreted cytokines, including interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α)], pathogen-associated molecular patterns (e.g., lipopolysaccharide), and damage-associated molecular patterns (e.g., HMGB1, heat shock proteins, ATP, fetuin-A, and oxidized low-density-lipoprotein) (41–45).
M1 macrophage markers include CD80, CD86, inducible nitric oxide synthase (iNOS), major histocompatibility complex (MHC)-II, Toll-like receptor (TLR)-2, and TLR-4. These cells release various cytokines and chemokines, such as TNF-α, IL-1α, IL-1β, IL-6, IL-12, CXC motif chemokine ligand (CXCL)9, CXCL10, and CXCL11, which exert positive feedback on unpolarized macrophages (41, 43, 46). NF-κB, STAT1, IRF3, and IRF5 signaling pathways are the main pathways involved in the regulation of an M1 polarization and transcriptional program, resulting in M1 powerful cytotoxic activities (41, 47).
M2 polarization occurs in response to downstream signals of cytokines such as IL-4, IL-13, IL-10, IL-33, and transforming growth factor-β (TGF-β) (31, 48). IL-4 and IL-13 directly induce M2 macrophage activation, whereas other cytokines (such as IL-33 and IL-25) amplify M2 macrophage activation by producing Th2 cytokines (49). M2 macrophages resolve inflammation via upregulation of anti-inflammatory mediators, such as IL-10, TGF-β, IL1-R type II, and IL-1Ra, and recruit Th2, Tregs, as well as eosinophils and basophils through C-C motif chemokine ligand (CCL)17, CCL18, CCL22, and CCL24 (50). They express markers such as the scavenger receptors CD206 and CD163, as well as fibrosis-related proteins like arginase-1, FIZZ1, chitinase 3 like-1 and -2 (YKL-40 in humans), CCL18, and IL-1β, which contribute to fibroblast activation and ECM accumulation in the lung (51–53). The M2 phenotype is notably driven and maintained by STAT3 and -6, peroxisome proliferator-activated receptor (PPAR)δ, and PPARγ signaling pathway (41, 49). Mechanistically, IL-4 stimulation leads to STAT6 phosphorylation, which activates the transcription of profibrotic target genes (54). KLF4, a member of the Krüppel-like factor family, has been shown to induce monocyte differentiation in vivo and to suppress inflammatory gene expression in macrophages, inducing an M2 phenotype (55, 56). Conversely, KLF4-deficient macrophages demonstrated increased proinflammatory gene expression and enhanced bactericidal activity, two features of M1-macrophages (56).
Epigenetic regulation of chromatin activity has also emerged as an important factor in polarized activation. Histone acetylation and methylation influence M1/M2 polarization. For instance, histone deacetylase 3 (HDAC3) has been shown to promote M1 polarization, and HDAC3-/- macrophages skew toward the M2 phenotype(57). In contrast, IL-4-induced expression of H3K27 demethylase Jumonji domain containing-protein 3 (Jmjd3) results in the upregulation of M2 genes and subsequently M2 polarization (58, 59). Posttranslational epigenetic regulation controlled by micro-RNAs (miRNA) represents an additional regulatory mechanism of macrophage polarization. For instance, miR-155 supports M1-driven inflammation following stimulation by TLR ligands, while it inhibits the M2 program by interfering with IL-13 and TGF-β signaling (14, 41, 60–62). miR-125b also enhances M1 polarization while miR-223, -124, and -125a-5p and Let-c promote a M2 program (63).
MONOCYTES AND MACROPHAGES IN PULMONARY FIBROSIS
Circulating Monocytes and Monocyte-Specific Genes as Biomarkers of PF Progression and Mortality
The contribution of monocytes to lung fibrosis is relatively poorly documented, compared to macrophages. However, the research interest in monocytes has been recently revived following translational studies reporting that a peripheral blood 52-gene expression signature classified patients with IPF into low- or high-risk groups for mortality (1, 22, 23). Cellular deconvolution of this 52-gene signature showed that monocytes represent the cellular source of the high-risk profile (23). This finding fueled further studies for the prognostic role of monocyte counts as markers of IPF mortality and decreased survival in other fibrotic disorders. Both pooled analysis of clinical trials and real-life data have demonstrated that increased monocyte counts are indeed linked to increased risk of IPF progression, hospitalization, and mortality (1, 24–26, 64).
Translational research studies showed that monocytes in patients with IPF are phenotypically different from age-matched controls, exhibiting a primed type I interferon pathway and markedly high serum levels of CSF-1, CCL-2, and IL-6 levels (65). Authors also performed single-cell RNA-sequencing analysis of cell suspensions from IPF lung tissues and healthy donors and showed a progression in transcriptomic states from monocytes (CD14+CD206neg/loCD69neg/lo) to early transitional macrophages (CD14+CD206lo, CD68lo), to later transitional macrophages (CD14+CD206lo, CD68mid-hi), to lung macrophages (CD206mid-hiCD68mid-hiCD14neg/lo) (65).
In addition to translational studies in IPF and other fibrotic disorders, murine models of PF demonstrated that both newly recruited monocyte-derived lung macrophages and long-lived lung resident AMs contribute to the progression of PF (33, 37, 66). Evren and colleagues (33) showed that extravasating CD14+ monocytes give rise to AM and IMs. The authors used MISTRG, a humanized mouse model, and adoptively transferred purified blood monocytes (CD14+CD16– monocytes) subsets into adult MISTRG mice and generated human lung macrophages with an AM phenotype (CD45+HLA-DR+CD11b+CD206+CD169+) 3 weeks posttransfer. In contrast, they showed that nonclassical CD14loCD16+ monocytes were confined to the lung vasculature and gave rise to a distinct population of pulmonary intravascular macrophages. Misharin and colleagues (67) showed that 5 days postadministration of bleomycin in mice, the number of interstitial macrophages (identified as CD64+Silgec F−) increases, whereas the number of AMs (CD64+Siglec F+) is reduced. In contrast, during the fibrotic phase (day 21 postbleomycin administration), the number of IMs decreases, and the number of AMs increases. The same group subsequently developed a lineage tracing system in mice to identify Mo-AMs and tissue-resident alveolar macrophages (TR-AMs) during the development of fibrosis and over the subsequent life span of the animal (37). They used a genetic lineage tracing system to show that Mo-AMs and TR-AMs play distinct roles during the development of lung fibrosis. They showed in the adenoviral TGF-β and bleomycin model of lung fibrosis that deletion of Mo-AM after their recruitment to the lung markedly attenuated the severity of fibrosis, whereas the deletion of TR-AM had no effect on fibrosis severity. These findings are bolstered by transcriptomic profiles of monocyte and macrophage populations over the course of bleomycin-induced fibrosis, which reveals substantial differences in gene expression between Mo-AMs and TR-AMs over the course of lung fibrosis. The authors also performed transcriptomic profiling of flow-sorted monocyte and macrophage populations from lung homogenates, and their results suggest that monocyte to AM differentiation represents a continuous downregulation of genes typically expressed in monocytes and upregulation of genes expressed in AMs. Taken together, their findings suggest that circulating monocytes transition to interstitial macrophages and Mo-AMs.
Monocytes contribute to both the adaptive and innate arms of the immune system. While they can initiate inflammatory responses upon injury, they also participate in normal and aberrant repair processes of injured tissue. For example, a subset of monocytes that displays granulocyte characteristics has been shown to participate in murine fibrosis. These Ceacam1+Msr1+Ly6C−F4/80−Mac1+ monocytes, named SatM (segregated-nucleus-containing atypical monocytes), are regulated by CCAAT/enhancer binding protein β (C/EBPβ), and C/EBPβ knockout mice do not develop lung fibrosis following bleomycin treatment. However, the adoptive transfer of SatM cells into C/EBPβ knockout mice restored fibrosis susceptibility (68). In addition, depletion of circulating monocytes during the progressive fibrotic phase has been shown to strikingly reduce the degree of fibrosis following lung injury in the bleomycin model of lung fibrosis. In particular, the depletion of a subset of circulating monocytes called Ly6Chi was followed by a reduction of lung macrophage populations, including M2 macrophages (69).
From Circulating Monocytes to Profibrotic Macrophages
Previous studies reported that the lungs of PF patients and mice exposed to bleomycin are infiltrated by macrophages, which are essential for disease development and progression (70–73). This mechanism seems to be regulated by fibroblast sensing and control of macrophage populations through YAP-mediated mechanotransduction signaling (74–76). Reyfman et al. (77) performed single-cell RNA sequencing (scRNA-seq) on normal and fibrotic (including IPF) lung explants and identified four distinct macrophage clusters. Two of these clusters originated largely from patients with fibrosis and displayed increased expression of fibrotic genes such as MARCK5, IL1RA, PLA2G7, matrix metalloproteinase 9 (MMP9), SPP1, and CHI3L1, which were not detected in normal tissues. Subsequent scRNA-seq studies identified three discrete macrophage subpopulations, which were all present in normal and fibrotic lungs. One had markers of monocyte-derived macrophages, and the other two were resident-like macrophage subpopulations that either expressed high FABP4 and INHBA (FABP4hi) or high SPP1 and MERTK (SPP1hi) (78). The presence of SPP1hi profibrotic macrophages was confirmed by Adams and colleagues (20). Another relevant finding is the identification of CX3CR1+ platelet-derived growth factor (PDGF)-AA+ transitional macrophages by scRNA-seq in fibrotic lungs from bleomycin-treated mice. These monocyte-derived, disease-associated macrophages transitioned to AMs and were localized to the fibrotic niche and exerted profibrotic effects, notably via secretion of PDGF-AA, a mitogen necessary for alteration of fibroblast phenotype. Ablation of CX3CR1+PDGF-AA+ macrophages protected mice from lung fibrosis (79). Interestingly, the authors report an increased expression of CX3CR1 in human fibrotic lungs and found that PDGF-AA+ macrophages were associated with fibrotic regions in the lungs (79). In a recent study, Ayaub et al. (80) identified an expanded population of macrophages (IPFeMΦ) in IPF biopsies using a combined scRNA-seq/mass cytometry (CyTOF)/flow cytometry approach. IPFeMΦ expressed a profibrotic signature compared to chronic obstructive pulmonary disease and healthy lung macrophages. The authors reported that these CD84++CD36++ macrophages displayed a hybrid transitional state between AMs and IMs with M2-like features and specifically expressed Mo-AM markers such as CD14 and APOE, supporting an origin from circulating monocytes (Fig. 1).
Molecular Mechanisms Controlling Macrophage Activation in PF
Both M1 and M2 macrophages participate in the development of lung injury and progression to PF. Lung injury promotes the recruitment of monocyte-derived macrophages that initiate an inflammatory response by producing iNOS and several cytokines (81). Persistent or repeated cycles of alveolar epithelial cell injury promote fibroblast deposition, recruitment of monocyte-derived lung macrophages, and the transition of macrophages to an M2 phenotype. M2 polarization is driven by Th2-secreted cytokines, among which IL-4 is well demonstrated to be an important promoter of the process (Fig. 2). In a bleomycin-induced lung fibrosis model, AMs highly express the Grb2-associated binder proteins Gab1 and Gab2, which mediate IL-4-induced M2 polarization by activating phosphatidylinositol 3-kinase (PI3K)/AKT and JAK1/STAT6 signaling pathways, respectively (82). Interestingly, IL-4/JAK1/STAT6-induced M2 polarization in bleomycin-treated mouse lungs is inhibited by tyrosine phosphatase Shp2, a known antifibrotic regulator in PF (83, 84). Another study demonstrated that signals transmitted from the extracellular matrix via the α4β1 integrin lead to the activation of Rac2, which in turn regulates alternative macrophage differentiation toward a profibrotic phenotype. Mice deficient in Rac2 are protected against bleomycin-induced PF and display diminished collagen deposition in association with lower expression of alternatively activated profibrotic macrophage markers (85). Mo-AMs with M2-like features are thought to be the main source of TGF-β in lung fibrosis (49, 86), but monocytes and monocyte-derived lung macrophages also release other profibrotic factors such as matrix metalloproteinases that are important for the activation, differentiation, and accumulation of fibroblasts, as well as epithelial-to-mesenchymal transition (87) (Fig. 2). Culture supernatants of AMs from IPF patients induce collagen production in normal lung fibroblasts, a mechanism partly dependent on CCL18 secretion, a marker of M2-like macrophage activation (76). In addition, IL-4-induced M2 macrophages secrete insulin-like growth factor-1 (IGF-1) abundantly, which in turn protects myofibroblast from apoptosis (88). Activated monocytes and macrophages also secrete growth factors such as PDGF and proangiogenic vascular endothelial growth factor, especially the vascular endothelial growth factor (VEGF)-A165b variant, both participating in the fibrotic process and activating signaling pathways that are targeted by Nintedanib (89–93).
Figure 2.
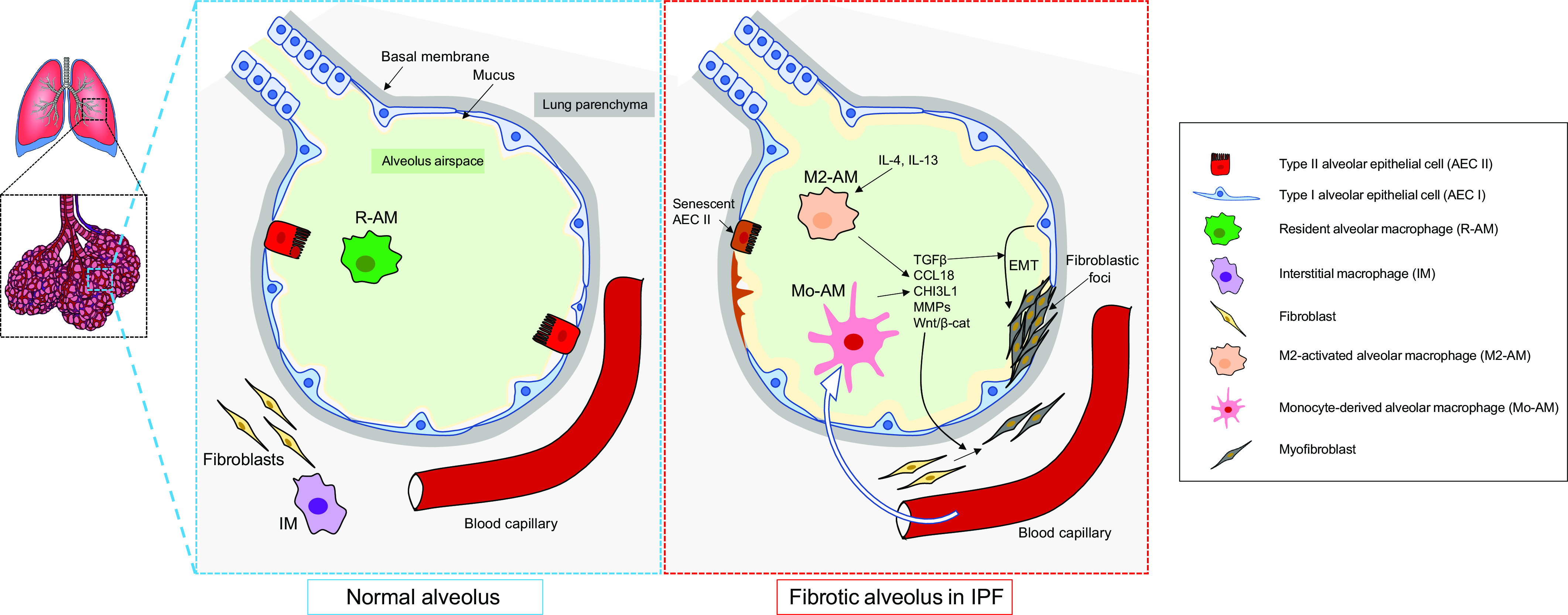
Recruitment and cytokine release by monocyte-derived alveolar macrophages and M2 alveolar macrophages in idiopathic pulmonary fibrosis (IPF). EMT, epithelial-to-mesenchymal transition; TGF-β, transforming growth factor-β. MMPs, matrix metalloproteinases. CCL18, C-C motif chemokine ligand 18.
Several other mechanisms have been associated with macrophage activation in PF. For example, adenosine, which is generated in excess during cellular stress and damage, is abundant in IPF lungs, and the activation of its receptor, ADORA2B, polarizes macrophages to a fibrotic phenotype (94, 95). Hypoxic conditions lead to hypoxia-inducible factor-1α upregulation, which in turn induces ADORAB2 expression in alternatively activated macrophages (96, 97). Macrophage activation in IPF lung is also related to dysregulated cellular metabolism, a mechanism that includes augmented glycolysis (98). The expression of the glucose transporter GLUT1 is increased in AMs from IPF patients, which enhances glucose intake and subsequent NADPH production (99, 100). NADPH then serves as a substrate of NADPH oxidase (NOX) and peroxynitrite OONO− production, a cytotoxic and tissue-damaging reactive oxygen species (ROS) found elevated in the lungs of IPF patients (101).
Mitochondrial oxidative stress and mitochondrial turnover are also directly linked to AM activation and PF. The expression of the mitochondrial calcium uniporter (MCU), which transports Ca2+ into the mitochondrial matrix to modulate metabolism, is increased in IPF AMs (102). MCU-mediated mitochondrial Ca2+ influx, as well as ROS and ATP production, modulate macrophage alternative activation to a profibrotic phenotype (103). Additionally, activation of macrophages toward a proinflammatory phenotype can cause impaired mitochondrial respiration and tricarboxylic acid cycle disruption, resulting in the accumulation of endogenous metabolites with potential immunomodulatory roles, such as the antifibrotic itaconate (104, 105). In IPF, decreased levels of itaconate were detected in the patients’ airways, as well as a downregulation of ACOD1, an enzyme that controls the synthesis of itaconate, in airway macrophages (104). In the bleomycin model, Acod−/− mice develop persistent fibrosis compared to their WT littermates, and higher expression of fibrosis-related genes was observed in Acod−/− airway M2-like macrophages (104).
Endoplasmic reticulum (ER) stress is believed to skew macrophage polarization toward either an M1 or M2 phenotype depending on the pathophysiological context. C/EBP homologous protein (CHOP), a marker of ER, is upregulated in lung macrophages of IPF patients and promotes an M2 program in macrophages through STAT6/PPARγ signaling (71). Iron metabolism has also been associated with macrophage activation during fibrosis. For example, Allden et al. (106) reported the expansion of a transferrin (CD71)-defective AM population in bronchoalveolar lavage (BAL) fluid and lung tissue from IPF patients with progressive fibrotic disease. CD71, which binds most of the free iron in the blood, has been described as a marker of mature macrophages. In IPF, CD71− AMs display functional and phenotypic immaturity and express profibrotic factors such as metalloproteases (MMP)-2 and -9 and plasminogen activator inhibitor 1 (PAI-1), a well-characterized TGF-β transcriptional target. Taken together, these data show the relevance and mechanistic implications of activated lung macrophages and their role in lung fibrosis.
How Do Macrophages Drive the Fibrotic Process?
Multiple studies have demonstrated the importance of M2-like Mo-AMs in lung fibrosis (107). In mice, the inhibition of M2 polarization protects mice from bleomycin-induced PF (108). In the same murine PF model, depletion and adoptive transfer of Mo-AMs protect or expose animals to PF, respectively (37, 68). However, how these cells precisely induce fibrosis in IPF needs to be addressed. Mo-AMs with M2-like features are thought to be the main source of TGF-β in PF (86, 109), but lung macrophages also release other profibrotic factors that are important for the activation, differentiation, and accumulation of fibroblasts, as well as epithelial-to-mesenchymal transition (EMT) (110) (Fig. 2).
Macrophages and the Extracellular Matrix
The extracellular matrix (ECM) plays a crucial role in regulating various cellular processes such as adhesion, migration, cell cycle, metabolism, and differentiation. During fibrosis, various enzymes including matrix metalloproteases (MMPs) are responsible for ECM degradation, leading to the formation of fibrotic niches (111). In IPF lungs, high levels of MMPs as well as tissue inhibitors of MMPs (TIMPs) are secreted by macrophages and result in dysregulated collagen turnover and abnormal matrix deposition (112). MMPs increase active TGF-β1 release from the ECM, which in turn regulates ECM deposition and degradation. In addition, overexpression of TGF-β1 has been found to reduce the antifibrotic activity of MMP1 and to induce the expression of tissue inhibitor of metalloproteinase 1 (TIMP1), resulting in an overall increase in ECM deposition. On the other hand, the profibrotic MMP7 has been found to be abundantly expressed in IPF patients and is localized on the surface of AMs (113). Various other macrophage-secreted MMPs have been found to contribute to or to resolve PF and macrophage polarization, including MMP-2, -7, -12, -13, -14, and -28 (114). In a recent article, Adams et al. (20) used scRNA-seq to establish a single-cell atlas of IPF, which uncovered the existence of a profibrotic IPF macrophage archetype among 18 distinct varieties of immune cells. As previously reported, the authors observed an increase in profibrotic and ECM remodeling genes such as SPP1, CHI3L1, CTSK, SPARC (osteonectin), GPC4, PALLD, and MMP9 (20, 78, 115). The metalloprotease MMP9, also called gelatinase B, is predominantly expressed by AMs, and its active form is abundant in BAL fluid from patients with rapidly progressive forms of IPF (116, 117). In addition, blood monocytes and lung macrophages are key cell types contributing to the elevated MMP-8 levels in IPF lungs, and macrophages in areas of mild as well as severe fibrosis robustly express MMP-8 (118). Culture supernatants of AMs from IPF patients induce collagen production in normal lung fibroblasts, a mechanism partly dependent on CCL18 secretion, a marker of M2-like macrophage activation (76). In addition, IL-4-induced M2 macrophages secrete IGF-1 abundantly, which in turn protects ECM-producing myofibroblast from apoptosis (88). M2-like macrophages secrete growth factors such as PDGF-AA and proangiogenic VEGF-A, both participating in the fibrotic process and activating signaling pathways that are targeted by Nintedanib, one of the two Food and Drug Administration-approved drugs in the treatment of IPF (93, 119, 120). IL-10, whose levels are found highly increased in lungs from IPF patients, generates a profibrotic Th2 microenvironment that involves M2 macrophage activation and fibrocyte recruitment likely in a CCL2/CCR2-dependent manner (121, 122).
Oxidative Stress, Abnormal Metabolism, and Cell Senescence
Pulmonary macrophages were found to promote IPF via the secretion of ROS (40). ROS exert a critical role in the activation of TGF-β, which in turn increases ROS production from mitochondria and NOX activation, forming a profibrotic positive feedback loop (123–125). Interestingly, an excessive number of iron-laden macrophages have been observed in IPF and are associated with oxidative stress and ROS production (126, 127). Yet, iron accumulation shapes macrophage polarization through different signaling pathways and is associated with diffuse vascular abnormalities in IPF patients (128–130). In addition, lipid metabolism seems to be involved in AM-induced IPF. Activated M2-AM uses fatty acid oxidation to increase the expression of arginase-1, which catalyzes the synthesis of ornithine from arginine. Ornithine then serves as an essential precursor for collagen during wound healing (131). Furthermore, nitrated fatty acids (NFAs), which are potent agonists of the antifibrotic nuclear receptor PPARγ expressed in AMs, have been shown to reverse bleomycin-induced lung fibrosis in mice. In AMs, NFA treatment upregulated MFG-E8 expression, a glycoprotein that targets collagen for uptake and lysosomal degradation by macrophages. In addition, NFA promotes myofibroblast dedifferentiation (132). Gao and colleagues (73) reported a potentially important role for netrin (NTN)-1-expressing macrophages in PF, including IPF. The authors showed that macrophage-derived NTN-1 remodeled adrenergic nerves and augmented noradrenaline release, thus promoting lung fibrosis. They found an increased number of NTN-1-expressing macrophages and norepinephrine enrichment in IPF lungs and observed that macrophage-specific deletion of Ntn-1-protected mice against bleomycin-induced PF (73).
IPF is an age-related disease involving cellular senescence, telomere shortening, epigenetic modifications, and mitochondrial dysfunction (12, 133). Aging causes phagocytotic cells to lose function, increasing basal innate immune signaling and decreasing cellular defense. In aging alveolar microenvironments, inflammatory factors such as TNF-α, IL-12, IL-1β, IL-6, IL-10, and IFN-γ are released by aging macrophages. Senescence of type II alveolar epithelial cells results in AM activation and p16 induction, promoting lung fibrogenesis (134). Higher levels of profibrotic IL-10 and macrophage migration inhibitory factor, related to telomere shortening and ECM deposition, are both present in fibrotic niches and BAL in IPF patients (135).
THERAPEUTIC CONSIDERATIONS AND CONCLUDING REMARKS
Monocytes and monocyte-specific genes have been shown to predict IPF mortality and poor disease outcomes; thus targeting these may represent a novel strategy that could improve the survival of patients with IPF and other forms of PF (1, 24, 136). A recent report demonstrated that administration of diphtheria toxin and conditional depletion of CD11b cells in CD11b-diphtheria toxin receptor mice potently suppressed bleomycin-induced PF (137). Antithetic experiments performed by others have shown that myeloid-specific deletion of activating transcription factor 6 alpha led to increases in CD11b+ macrophage subpopulations and worse PF in the bleomycin model (138). Targeting several proteins expressed in macrophages has been shown to ameliorate PF in murine models, particularly when liposomal chlodronate is used to deplete Mo-AM and M2 macrophage populations validating the potential of these cells as therapeutic targets in PF (37, 71, 139, 140). Other compounds targeting pathogenic macrophages such as SHP-1 agonists have been shown to alleviate PF in murine models as well (141).
In addition to macrophage depletion, macrophage reprogramming seems to be a novel and promising therapeutic approach that may be applicable to IPF. Recently, Ahangari and colleagues (142) demonstrated that specific genetic ablation of miR-33 in macrophages protected against bleomycin-induced pulmonary fibrosis by improving mitochondrial homeostasis and increasing autophagy while decreasing inflammatory response after bleomycin injury. The authors also demonstrated that a novel model for pharmacological inhibition of miR-33 in macrophages, via administration of anti-miR-33 peptide nucleic acids (PNA-33), showed attenuation of fibrosis in different in vivo and ex vivo mice and human models of PF (142). In addition, a recent report demonstrated that nanoparticles efficiently delivered small-interfering RNA against TGF-β1, targeting de novo profibrotic Mo-AMs and leading to decreased murine lung fibrosis (143). The therapeutic effect of macrophage reprogramming has been shown by others as well. For instance, Zhang and colleagues (53) created a folate-targeted TLR7 agonist (FA-TLR7-54) that selectively accumulated in profibrotic macrophages and suppressed fibrosis-inducing cytokine production. The authors demonstrate that FA-TLR7-54 reprogrammed M2-like fibrosis-inducing macrophages into fibrosis-suppressing macrophages, resulting in dramatic declines in profibrotic cytokine release and measurements of murine PF (53). Others have shown that macrophages loaded with liposomal dexamethasone and delivered to bleomycin-treated mice reduced murine PF (144). Similar results were seen with the pharmacological blockade of M-CSFR signaling, which led to the disappearance of Mo-AMs and ameliorated fibrosis during asbestos-induced fibrosis (145). Taken together, these results demonstrate that monocytes, Mo-AMs, and profibrotic macrophages are not only mechanistically relevant for PF development and progression, but targeting these key immune cells leads to a reduction in murine PF and may result in therapies that could improve the survival of patients with IPF and other forms of PF. A recent study showed that intravenous administration of mesenchymal stem cells ameliorated pulmonary fibrosis in the bleomycin model through inhibition of monocyte differentiation into M2 macrophages (146). This might revive the interest for future clinical trials studying the effect of stem cells in IPF (147, 148). With regards to other clinical trials, recombinant human pentraxin 2, a potent inhibitor of monocyte differentiation, failed to show efficacy in the recent phase 3 trial, despite previous promising results in the phase 2 trial (149–151). Galectin 3 inhibition, which might affect monocytes’ profile as well, has led to promising data so far, and further results from clinical trials are greatly anticipated (151–153). Further future studies aiming at translating the aforementioned findings into clinical trials are required to determine the clinical applicability of targeting monocytes and lung macrophages in PF.
GRANTS
This work was supported by the Ubben Family Fund (#250392).
DISCLAIMERS
The funders did not have any role in paper design, data collection, data analysis, interpretation and writing of the paper.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.Y.P., T.K., and J.D.H.-M. conceived and designed research; C.Y.P., T.K., and J.D.H.-M. analyzed data; C.Y.P., T.K., and J.D.H.-M. interpreted results of experiments; C.Y.P., T.K., and J.D.H.-M. prepared figures; C.Y.P., T.K., and J.D.H.-M. drafted manuscript; C.Y.P., T.K., and J.D.H.-M. edited and revised manuscript; C.Y.P., T.K., and J.D.H.-M. approved final version of manuscript.
REFERENCES
- 1. Karampitsakos T, Juan-Guardela BM, Tzouvelekis A, Herazo-Maya JD. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine 95: 104766, 2023. doi: 10.1016/j.ebiom.2023.104766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, Egan JJ, Lambrecht BN, Lories R, Parfrey H, Prasse A, Robalo-Cordeiro C, Verbeken E, Verschakelen JA, Wells AU, Verleden GM. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J 41: 1207–1218, 2013.doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 3. Spagnolo P, Sverzellati N, Rossi G, Cavazza A, Tzouvelekis A, Crestani B, Vancheri C. Idiopathic pulmonary fibrosis: an update. Ann Med 47: 15–27, 2015. doi: 10.3109/07853890.2014.982165. [DOI] [PubMed] [Google Scholar]
- 4. Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5: e62, 2008. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, , et al. Future directions in idiopathic pulmonary fibrosis research: an NHLBI workshop report. Am J Respir Crit Care Med . 189: 214–222, 2014. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzouvelekis A, Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol 93: 159–170, 2015. doi: 10.1139/bcb-2014-0126. [DOI] [PubMed] [Google Scholar]
- 7. Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182: 220–229, 2010. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 287–294, 2011. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589–1597, 2010. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, McMillan M, Kahn M, Kaminski N. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol 47: 879–887, 2012. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papaioannou O, Karampitsakos T, Barbayianni I, Chrysikos S, Xylourgidis N, Tzilas V, Bouros D, Aidinis V, Tzouvelekis A. Metabolic disorders in chronic lung diseases. Front Med (Lausanne) 4: 246, 2017. doi: 10.3389/fmed.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, de Castro JP, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo ED, Gan Y, Graham M, Liu X, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 24: 39–49, 2018. doi: 10.1038/nm.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, Bouros D, Tzouvelekis A. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 45: 1–10, 2017.doi: 10.1016/j.pupt.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 14. Karampitsakos T, Woolard T, Bouros D, Tzouvelekis A. Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur J Pharmacol 808: 35–43, 2017. doi: 10.1016/j.ejphar.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 15. Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, Duncan SR. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One 5: e8959, 2010. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, Bouros D. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179: 1121–1130, 2009. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 17. Xylourgidis N, Min K, Ahangari F, Yu G, Herazo-Maya JD, Karampitsakos T, Aidinis V, Binzenhöfer L, Bouros D, Bennett AM, Kaminski N, Tzouvelekis A. Role of dual-specificity protein phosphatase DUSP10/MKP-5 in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 317: L678–L689, 2019. doi: 10.1152/ajplung.00264.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karampitsakos T, Vraka A, Bouros D, Liossis SN, Tzouvelekis A. Biologic treatments in interstitial lung diseases. Front Med (Lausanne) 6: 41, 2019. doi: 10.3389/fmed.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ntatsoulis K, Karampitsakos T, Tsitoura E, Stylianaki EA, Matralis AN, Tzouvelekis A, Antoniou K, Aidinis V. Commonalities between ARDS, pulmonary fibrosis and COVID-19: the potential of autotaxin as a therapeutic target. Front Immunol 12: 687397, 2021. doi: 10.3389/fimmu.2021.687397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, Chu SG, Raby BA, DeIuliis G, Januszyk M, Duan Q, Arnett HA, Siddiqui A, Washko GR, Homer R, Yan X, Rosas IO, Kaminski N. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6: eaba1983, 2020. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karampitsakos T, Papaioannou O, Katsaras M, Sampsonas F, Tzouvelekis A. Interstitial lung diseases and the impact of gender. Clin Chest Med 42: 531–541, 2021. doi: 10.1016/j.ccm.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 22. Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, Huang Y, Vij R, Lindell KO, Xue J, Gibson KF, Shapiro SD, Garcia JG, Kaminski N. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med 5: 205ra136, 2013. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herazo-Maya JD, Sun J, Molyneaux PL, Li Q, Villalba JA, Tzouvelekis A, Lynn H, Juan-Guardela BM, Risquez C, Osorio JC, Yan X, Michel G, Aurelien N, Lindell KO, Klesen MJ, Moffatt MF, Cookson WO, Zhang Y, Garcia JG, Noth I, Prasse A, Bar-Joseph Z, Gibson KF, Zhao H, Herzog EL, Rosas IO, Maher TM, Kaminski N. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med 5: 857–868, 2017. doi: 10.1016/s2213-2600(17)30349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott MK, Quinn K, Li Q, Carroll R, Warsinske H, Vallania F, Chen S, Carns MA, Aren K, Sun J, Koloms K, Lee J, Baral J, Kropski J, Zhao H, Herzog E, Martinez FJ, Moore BB, Hinchcliff M, Denny J, Kaminski N, Herazo-Maya JD, Shah NH, Khatri P. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir Med 7: 497–508, 2019. doi: 10.1016/s2213-2600(18)30508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karampitsakos T, Torrisi S, Antoniou K, Manali E, Korbila I, Papaioannou O, , et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with idiopathic pulmonary fibrosis. Respir Res 22: 140, 2021. doi: 10.1186/s12931-021-01725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kreuter M, Lee JS, Tzouvelekis A, Oldham JM, Molyneaux PL, Weycker D, Atwood M, Kirchgaessler KU, Maher TM. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 204: 74–81, 2021.doi: 10.1164/rccm.202003-0669OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Juan Guardela BM, Sun J, Zhang T, Xu B, Balnis J, Huang Y, Ma SF, Molyneaux PL, Maher TM, Noth I, Michaud G, Jaitovich A, Herazo-Maya JD. 50-gene risk profiles in peripheral blood predict COVID-19 outcomes: a retrospective, multicenter cohort study. EBioMedicine 69: 103439, 2021. doi: 10.1016/j.ebiom.2021.103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karampitsakos T, Sampsonas F, Herazo-Maya JD, Tzouvelekis A. Management of patients with idiopathic pulmonary fibrosis and lung cancer: challenges in clinical practice. Curr Opin Pulm Med 29: 416–426, 2023. doi: 10.1097/mcp.0000000000000977. [DOI] [PubMed] [Google Scholar]
- 29. Karampitsakos T, Spagnolo P, Mogulkoc N, Wuyts WA, Tomassetti S, Bendstrup E, Molina-Molina M, Manali ED, Unat ÖS, Bonella F, Kahn N, Kolilekas L, Rosi E, Gori L, Ravaglia C, Poletti V, Daniil Z, Prior TS, Papanikolaou IC, Aso S, Tryfon S, Papakosta D, Tzilas V, Balestro E, Papiris S, Antoniou K, Bouros D, Wells A, Kreuter M, Tzouvelekis A. Lung cancer in patients with idiopathic pulmonary fibrosis: a retrospective multicentre study in Europe. Respirology 28: 56–65, 2023. doi: 10.1111/resp.14363. [DOI] [PubMed] [Google Scholar]
- 30. Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Muller-Quernheim J, Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol 137: 89–101, 2010. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 31. Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med (Lausanne) 5: 43, 2018. doi: 10.3389/fmed.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evren E, Ringqvist E, Tripathi KP, Sleiers N, Rives IC, Alisjahbana A, Gao Y, Sarhan D, Halle T, Sorini C, Lepzien R, Marquardt N, Michaelsson J, Smed-Sorensen A, Botling J, Karlsson MC, Villablanca EJ, Willinger T. Distinct developmental pathways from blood monocytes generate human lung macrophage diversity. Immunity 54: 259–275.e7, 2021.doi: 10.1016/j.immuni.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 34. Tarling JD, Lin HS, Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. J Leukoc Biol 42: 443–446, 1987. doi: 10.1002/jlb.42.5.443. [DOI] [PubMed] [Google Scholar]
- 35. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210: 1977–1992, 2013. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, , et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214: 2387–2404, 2017. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liegeois M, Legrand C, Desmet CJ, Marichal T, Bureau F. The interstitial macrophage: a long-neglected piece in the puzzle of lung immunity. Cell Immunol 330: 91–96, 2018. doi: 10.1016/j.cellimm.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 39. Dehring DJ, Wismar BL. Intravascular macrophages in pulmonary capillaries of humans. Am Rev Respir Dis 139: 1027–1029, 1989. doi: 10.1164/ajrccm/139.4.1027. [DOI] [PubMed] [Google Scholar]
- 40. Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res 19: 170, 2018. doi: 10.1186/s12931-018-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol 27: 237–248, 2015. doi: 10.1016/j.smim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 42. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci 72: 4111–4126, 2015. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol 308: F69–F75, 2015. doi: 10.1152/ajprenal.00484.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis 214: 345–349, 2011. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 46. Murray PJ. Macrophage polarization. Annu Rev Physiol 79: 541–566, 2017. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 47. Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 12: 231–238, 2011. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 48. Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY, McSharry C, Xu D. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol 134: 1422–1432.e11, 2014. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 50. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 51. Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol 6: 602, 2015.doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Y, Peng H, Sun H, Peng X, Tang C, Gan Y, Chen X, Mathur A, Hu B, Slade MD, Montgomery RR, Shaw AC, Homer RJ, White ES, Lee CM, Moore MW, Gulati M, Lee CG, Elias JA, Herzog EL. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci Transl Med 6: 240ra76, 2014. doi: 10.1126/scitranslmed.3007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang F, Ayaub EA, Wang B, Puchulu-Campanella E, Li YH, Hettiarachchi SU, Lindeman SD, Luo Q, Rout S, Srinivasarao M, Cox A, Tsoyi K, Nickerson-Nutter C, Rosas IO, Low PS. Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis. EMBO Mol Med 12: e12034, 2020.doi: 10.15252/emmm.202012034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol 172: 7565–7573, 2004. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 55. Alder JK, Georgantas RW 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol 180: 5645–5652, 2008. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest 121: 2736–2749, 2011. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mullican SE, Gaddis CA, Alenghat T, Nair MG, Giacomin PR, Everett LJ, Feng D, Steger DJ, Schug J, Artis D, Lazar MA. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev 25: 2480–2488, 2011. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 11: 936–944, 2010. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 59. Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, Kunkel SL. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 114: 3244–3254, 2009. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem 286: 1786–1794, 2011. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, Zen K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol 4: 341–343, 2012. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 62. Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta}. J Biol Chem 285: 41328–41336, 2010. doi: 10.1074/jbc.M110.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kishore A, Petrek M. Roles of macrophage polarization and macrophage-derived miRNAs in pulmonary fibrosis. Front Immunol 12: 678457, 2021. doi: 10.3389/fimmu.2021.678457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Teoh AK, Jo HE, Chambers DC, Symons K, Walters EH, Goh NS, Glaspole I, Cooper W, Reynolds P, Moodley Y, Corte TJ. Blood monocyte counts as a potential prognostic marker for idiopathic pulmonary fibrosis: analysis from the Australian IPF registry. Eur Respir J 55: 1901855, 2020. doi: 10.1183/13993003.01855-2019. [DOI] [PubMed] [Google Scholar]
- 65. Fraser E, Denney L, Antanaviciute A, Blirando K, Vuppusetty C, Zheng Y, Repapi E, Iotchkova V, Taylor S, Ashley N, St Noble V, Benamore R, Hoyles R, Clelland C, Rastrick JM, Hardman CS, Alham NK, Rigby RE, Simmons A, Rehwinkel J, Ho LP. Multi-modal characterization of monocytes in idiopathic pulmonary fibrosis reveals a primed type I interferon immune phenotype. Front Immunol 12: 623430, 2021. doi: 10.3389/fimmu.2021.623430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Zhang X, Wang J, Yang F, Luo W, Huang J, Chen M, Wang S, Li C, Zhang W, Chao J. ZC3H4 regulates infiltrating monocytes, attenuating pulmonary fibrosis through IL-10. Respir Res 23: 204, 2022. doi: 10.1186/s12931-022-02134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49: 503–510, 2013.doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, Minowa Y, Fukushima K, Ebina I, Yoshioka Y, Kumanogoh A, Akira S. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 541: 96–101, 2017. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 69. Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, Hirani N, Gauldie J, Iredale JP, Sethi T, Forbes SJ. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med 184: 569–581, 2011. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 70. Ji WJ, Ma YQ, Zhou X, Zhang YD, Lu RY, Sun HY, Guo ZZ, Zhang Z, Li YM, Wei LQ. Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury. J Immunol Methods 403: 7–16, 2014. doi: 10.1016/j.jim.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 71. Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, He X, Cheng Z, Ao Q, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Ning Q, Xiang X, Xiong W, Wang CY, Xu Y. Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther 24: 915–925, 2016. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hou J, Shi J, Chen L, Lv Z, Chen X, Cao H, Xiang Z, Han X. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun Signal 16: 89, 2018. doi: 10.1186/s12964-018-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gao R, Peng X, Perry C, Sun H, Ntokou A, Ryu C, Gomez JL, Reeves BC, Walia A, Kaminski N, Neumark N, Ishikawa G, Black KE, Hariri LP, Moore MW, Gulati M, Homer RJ, Greif DM, Eltzschig HK, Herzog EL. Macrophage-derived netrin-1 drives adrenergic nerve-associated lung fibrosis. J Clin Invest 131: e136542, 2021. doi: 10.1172/JCI136542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meli VS, Atcha H, Veerasubramanian PK, Nagalla RR, Luu TU, Chen EY, Guerrero-Juarez CF, Yamaga K, Pandori W, Hsieh JY, Downing TL, Fruman DA, Lodoen MB, Plikus MV, Wang W, Liu WF. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci Adv 6: eabb8471, 2020. doi: 10.1126/sciadv.abb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou X, Franklin RA, Adler M, Carter TS, Condiff E, Adams TS, Pope SD, Philip NH, Meizlish ML, Kaminski N, Medzhitov R. Microenvironmental sensing by fibroblasts controls macrophage population size. Proc Natl Acad Sci U S A 119: e2205360119, 2022. doi: 10.1073/pnas.2205360119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Muller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med 173: 781–792, 2006. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 77. Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, , et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199: 1517–1536, 2019. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, Jiang Y, Kass DJ, Gibson K, Chen W, Mora A, Benos PV, Rojas M, Lafyatis R. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J 54: 1802441, 2019.doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, Chak S, Naikawadi RP, Wolters PJ, Abate AR, Butte AJ, Bhattacharya M. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 20: 163–172, 2019. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ayaub E, Poli S, Ng J, Adams T, Schupp J, Quesada-Arias L, Poli F, Cosme C, Robertson M, Martinez-Manzano J, Liang X, Villalba J, Lederer J, Chu S, Raby B, Washko G, Coarfa C, Perrella M, El-Chemaly S, Kaminski N, Rosas I. Single cell RNA-seq and mass cytometry reveals a novel and a targetable population of macrophages in idiopathic pulmonary fibrosis. bioRxiv 2021.01.04.425268, 2021.doi: 10.1101/2021.01.04.425268. [DOI]
- 81. Xue Q, Yan Y, Zhang R, Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci 19: 3805, 2018. doi: 10.3390/ijms19123805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Guo X, Li T, Xu Y, Xu X, Zhu Z, Zhang Y, Xu J, Xu K, Cheng H, Zhang X, Ke Y. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J Biol Chem 292: 14003–14015, 2017. doi: 10.1074/jbc.M117.802066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tzouvelekis A, Yu G, Lino Cardenas CL, Herazo-Maya JD, Wang R, Woolard T, Zhang Y, Sakamoto K, Lee H, Yi JS, DeIuliis G, Xylourgidis N, Ahangari F, Lee PJ, Aidinis V, Herzog EL, Homer R, Bennett AM, Kaminski N. SH2 domain-containing phosphatase-2 is a novel antifibrotic regulator in pulmonary fibrosis. Am J Respir Crit Care Med 195: 500–514, 2017.doi: 10.1164/rccm.201602-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tao B, Jin W, Xu J, Liang Z, Yao J, Zhang Y, Wang K, Cheng H, Zhang X, Ke Y. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol 193: 2801–2811, 2014. doi: 10.4049/jimmunol.1303463. [DOI] [PubMed] [Google Scholar]
- 85. Joshi S, Singh AR, Wong SS, Zulcic M, Jiang M, Pardo A, Selman M, Hagood JS, Durden DL. Rac2 is required for alternative macrophage activation and bleomycin induced pulmonary fibrosis; a macrophage autonomous phenotype. PLoS One 12: e0182851, 2017. doi: 10.1371/journal.pone.0182851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med 170: 727–737, 1989. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res 17: 23, 2016.doi: 10.1186/s12931-016-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wynes MW, Frankel SK, Riches DW. IL-4-induced macrophage-derived IGF-I protects myofibroblasts from apoptosis following growth factor withdrawal. J Leukoc Biol 76: 1019–1027, 2004.doi: 10.1189/jlb.0504288. [DOI] [PubMed] [Google Scholar]
- 89. Martinet Y, Rom WN, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med 317: 202–209, 1987. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- 90. Antoniades HN, Bravo MA, Avila RE, Galanopoulos T, Neville-Golden J, Maxwell M, Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest 86: 1055–1064, 1990. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Barratt SL, Blythe T, Jarrett C, Ourradi K, Shelley-Fraser G, Day MJ, Qiu Y, Harper S, Maher TM, Oltean S, Hames TJ, Scotton CJ, Welsh GI, Bates DO, Millar AB. Differential expression of VEGF-Axxx isoforms is critical for development of pulmonary fibrosis. Am J Respir Crit Care Med 196: 479–493, 2017. doi: 10.1164/rccm.201603-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sangiuolo F, Puxeddu E, Pezzuto G, Cavalli F, Longo G, Comandini A, Di Pierro D, Pallante M, Sergiacomi G, Simonetti G, Zompatori M, Orlandi A, Magrini A, Amicosante M, Mariani F, Losi M, Fraboni D, Bisetti A, Saltini C. HFE gene variants and iron-induced oxygen radical generation in idiopathic pulmonary fibrosis. Eur Respir J 45: 483–490, 2015.doi: 10.1183/09031936.00104814. [DOI] [PubMed] [Google Scholar]
- 93. Richeldi L, Du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR, INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 94. Karmouty-Quintana H, Philip K, Acero LF, Chen NY, Weng T, Molina JG, Luo F, Davies J, Le NB, Bunge I, Volcik KA, Le TT, Johnston RA, Xia Y, Eltzschig HK, Blackburn MR. Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J 29: 50–60, 2015.doi: 10.1096/fj.14-260182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5: e9224, 2010.doi: 10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Philip K, Mills TW, Davies J, Chen NY, Karmouty-Quintana H, Luo F, Molina JG, Amione-Guerra J, Sinha N, Guha A, Eltzschig HK, Blackburn MR. HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis. FASEB J 31: 4745–4758, 2017. doi: 10.1096/fj.201700219R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cox P, Rinderknecht A, Blanco E. Masticatory biomechanics of the largest fossil rodent. FASEB J 29: 865–867, 2015. doi: 10.1096/fasebj.29.1_supplement.865.7. [DOI] [Google Scholar]
- 98. Xie N, Cui H, Ge J, Banerjee S, Guo S, Dubey S, Abraham E, Liu RM, Liu G. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 313: L834–L844, 2017. doi: 10.1152/ajplung.00235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. El-Chemaly S, Malide D, Yao J, Nathan SD, Rosas IO, Gahl WA, Moss J, Gochuico BR. Glucose transporter-1 distribution in fibrotic lung disease: association with [18F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest 143: 1685–1691, 2013. doi: 10.1378/chest.12-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, Metallo CM. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55: 253–263, 2014. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 1763–1769, 1997.doi: 10.1164/ajrccm.155.5.9154889. [DOI] [PubMed] [Google Scholar]
- 102. Gu L, Larson Casey JL, Andrabi SA, Lee JH, Meza-Perez S, Randall TD, Carter AB. Mitochondrial calcium uniporter regulates PGC-1alpha expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol 26: 101307, 2019. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gu L, Larson-Casey JL, Carter AB. Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization. FASEB J 31: 3072–3083, 2017. doi: 10.1096/fj.201601371R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ogger PP, Albers GJ, Hewitt RJ, O'Sullivan BJ, Powell JE, Calamita E, Ghai P, Walker SA, McErlean P, Saunders P, Kingston S, Molyneaux PL, Halket JM, Gray R, Chambers DC, Maher TM, Lloyd CM, Byrne AJ. Itaconate controls the severity of pulmonary fibrosis. Sci Immunol 5: eabc1884, 2020. doi: 10.1126/sciimmunol.abc1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, , et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556: 113–117, 2018. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Allden SJ, Ogger PP, Ghai P, McErlean P, Hewitt R, Toshner R, Walker SA, Saunders P, Kingston S, Molyneaux PL, Maher TM, Lloyd CM, Byrne AJ. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 200: 209–219, 2019. doi: 10.1164/rccm.201809-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ogawa T, Shichino S, Ueha S, Matsushima K. Macrophages in lung fibrosis. Int Immunol 33: 665–671, 2021. doi: 10.1093/intimm/dxab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Arizmendi N, Puttagunta L, Chung KL, Davidson C, Rey-Parra J, Chao DV, Thebaud B, Lacy P, Vliagoftis H. Rac2 is involved in bleomycin-induced lung inflammation leading to pulmonary fibrosis. Respir Res 15: 71, 2014. doi: 10.1186/1465-9921-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 110. Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 19: 32, 2018. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhao X, Chen J, Sun H, Zhang Y, Zou D. New insights into fibrosis from the ECM degradation perspective: the macrophage-MMP-ECM interaction. Cell Biosci 12: 117, 2022. doi: 10.1186/s13578-022-00856-w. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J 38: 1461–1467, 2011. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 113. Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, Nakamura M, Tasaka S, Hasegawa N, Aikawa N, Ishizaka A, Okada Y. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med 134: 1136–1142, 2010. doi: 10.5858/2009-0144-OA.1. [DOI] [PubMed] [Google Scholar]
- 114. Sari E, He C, Margaroli C. Plasticity toward rigidity: a macrophage conundrum in pulmonary fibrosis. Int J Mol Sci 23: 11443, 2022. doi: 10.3390/ijms231911443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reyfman PA, Gottardi CJ. Idiopathic pulmonary fibrosis and lung cancer: finding similarities within differences. Am J Respir Cell Mol Biol 61: 667–668, 2019. doi: 10.1165/rcmb.2019-0172ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McKeown S, Richter AG, O'Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J 33: 77–84, 2009. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 117. Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 162: 1949–1956, 2000. doi: 10.1164/ajrccm.162.5.9906096. [DOI] [PubMed] [Google Scholar]
- 118. Craig VJ, Polverino F, Laucho-Contreras ME, Shi Y, Liu Y, Osorio JC, Tesfaigzi Y, Pinto-Plata V, Gochuico BR, Rosas IO, Owen CA. Mononuclear phagocytes and airway epithelial cells: novel sources of matrix metalloproteinase-8 (MMP-8) in patients with idiopathic pulmonary fibrosis. PloS one 9: e97485, 2014. doi: 10.1371/journal.pone.0097485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Klüglich M, Du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 365: 1079–1087, 2011. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 120. Richeldi L, Kreuter M, Selman M, Crestani B, Kirsten AM, Wuyts WA, Xu Z, Bernois K, Stowasser S, Quaresma M, Costabel U. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: results from the TOMORROW trial and its open-label extension. Thorax 73: 581–583, 2018. doi: 10.1136/thoraxjnl-2016-209701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. She YX, Yu QY, Tang XX. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov 7: 52, 2021. doi: 10.1038/s41420-021-00437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol 300: L341–L353, 2011. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ogger PP, Byrne AJ. Lung fibrosis enters the iron age(†). J Pathol 252: 1–3, 2020. doi: 10.1002/path.5489. [DOI] [PubMed] [Google Scholar]
- 124. Veith A, Moorthy B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr Opin Toxicol 7: 44–51, 2018. doi: 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal 31: 1092–1115, 2019. doi: 10.1089/ars.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Puxeddu E, Comandini A, Cavalli F, Pezzuto G, D'Ambrosio C, Senis L, Paci M, Curradi G, Sergiacomi GL, Saltini C. Iron laden macrophages in idiopathic pulmonary fibrosis: the telltale of occult alveolar hemorrhage? Pulm Pharmacol Ther 28: 35–40, 2014. doi: 10.1016/j.pupt.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 127. Lee J, Arisi I, Puxeddu E, Mramba LK, Amicosante M, Swaisgood CM, Pallante M, Brantly ML, Sköld CM, Saltini C. Bronchoalveolar lavage (BAL) cells in idiopathic pulmonary fibrosis express a complex pro-inflammatory, pro-repair, angiogenic activation pattern, likely associated with macrophage iron accumulation. PLoS One 13: e0194803, 2018. doi: 10.1371/journal.pone.0194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim J, Wessling-Resnick M. The role of iron metabolism in lung inflammation and injury. J Allergy Ther 3: 004, 2012. doi: 10.4172/2155-6121.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Colombat M, Mal H, Groussard O, Capron F, Thabut G, Jebrak G, Brugiere O, Dauriat G, Castier Y, Leseche G, Fournier M. Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: Histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum Pathol 38: 60–65, 2007. doi: 10.1016/j.humpath.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 130. Xia L, Guo L, Kang J, Yang Y, Yao Y, Xia W, Sun R, Zhang S, Li W, Gao Y, Chen H, Li Z, Yang J, Lu S, Wang Y. Predictable roles of peripheral IgM memory B cells for the responses to anti-PD-1 monotherapy against advanced non-small cell lung cancer. Front Immunol 12: 759217, 2021. doi: 10.3389/fimmu.2021.759217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5: 532, 2014. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Reddy AT, Lakshmi SP, Zhang Y, Reddy RC. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J 28: 5299–5310, 2014. doi: 10.1096/fj.14-256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–38, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rana T, Jiang C, Liu G, Miyata T, Antony V, Thannickal VJ, Liu RM. PAI-1 Regulation of TGF-beta1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am J Respir Cell Mol Biol 62: 319–330, 2020. doi: 10.1165/rcmb.2019-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bargagli E, Olivieri C, Nikiforakis N, Cintorino M, Magi B, Perari MG, Vagaggini C, Spina D, Prasse A, Rottoli P. Analysis of macrophage migration inhibitory factor (MIF) in patients with idiopathic pulmonary fibrosis. Respir Physiol Neurobiol 167: 261–267, 2009. doi: 10.1016/j.resp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 136. Unterman A, Zhao AY, Neumark N, Schupp JC, Ahangari F, Cosme C, Sharma P, Flint J, Stein Y, Ryu C, Ishikawa G, Sumida TS, Gomez JL, Herazo-Maya J, Cruz CS, Herzog EL, Kaminski N. Single-cell profiling reveals immune aberrations in progressive idiopathic pulmonary fibrosis. medRxiv 2023.04.29.23289296, 2023. doi: 10.1101/2023.04.29.23289296. [DOI] [PMC free article] [PubMed]
- 137. Wan X, Xiao Y, Tian X, Lu Y, Chu H. Selective depletion of CD11b-positive monocytes/macrophages potently suppresses bleomycin-induced pulmonary fibrosis. Int Immunopharmacol 114: 109570, 2023. doi: 10.1016/j.intimp.2022.109570. [DOI] [PubMed] [Google Scholar]
- 138. Mekhael O, Revill SD, Hayat AI, Cass SP, MacDonald K, Vierhout M, Ayoub A, Reihani A, Padwal M, Imani J, Ayaub E, Yousof T, Dvorkin-Gheva A, Rullo A, Hirota JA, Richards CD, Bridgewater D, Stampfli MR, Hambly N, Naqvi A, Kolb MR, Ask K. Myeloid-specific deletion of activating transcription factor 6 alpha increases CD11b(+) macrophage subpopulations and aggravates lung fibrosis. Immunol Cell Biol 101: 412–427, 2023. doi: 10.1111/imcb.12637. [DOI] [PubMed] [Google Scholar]
- 139. Li Q, Cheng Y, Zhang Z, Bi Z, Ma X, Wei Y, Wei X. Inhibition of ROCK ameliorates pulmonary fibrosis by suppressing M2 macrophage polarisation through phosphorylation of STAT3. Clin Transl Med 12: e1036, 2022. doi: 10.1002/ctm2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, Gulati M, Homer RJ, Russell T, van Rooijen N, Elias JA, Hogaboam CM, Herzog EL. TGF-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol 43: 154–162, 2011. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 141. Hong SY, Lu YT, Chen SY, Hsu CF, Lu YC, Wang CY, Huang KL. Targeting pathogenic macrophages by the application of SHP-1 agonists reduces inflammation and alleviates pulmonary fibrosis. Cell Death Dis 14: 352, 2023. doi: 10.1038/s41419-023-05876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ahangari F, Price NL, Malik S, Chioccioli M, Barnthaler T, Adams TS, Kim J, Pradeep SP, Ding S, Cosmos C Jr, Rose KS, McDonough JE, Aurelien NR, Ibarra G, Omote N, Schupp JC, DeIuliis G, Villalba Nunez JA, Sharma L, Ryu C, Dela Cruz CS, Liu X, Prasse A, Rosas I, Bahal R, Fernandez-Hernando C, Kaminski N. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI Insight 8: e158100, 2023. doi: 10.1172/jci.insight.158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Singh A, Chakraborty S, Wong SW, Hefner NA, Stuart A, Qadir AS, Mukhopadhyay A, Bachmaier K, Shin JW, Rehman J, Malik AB. Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis. Proc Natl Acad Sci U S A 119: e2121098119, 2022. doi: 10.1073/pnas.2121098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sang X, Wang Y, Xue Z, Qi D, Fan G, Tian F, Zhu Y, Yang J. Macrophage-targeted lung delivery of dexamethasone improves pulmonary fibrosis therapy via regulating the immune microenvironment. Front Immunol 12: 613907, 2021. doi: 10.3389/fimmu.2021.613907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Joshi N, Watanabe S, Verma R, Jablonski RP, Chen CI, Cheresh P, Markov NS, Reyfman PA, McQuattie-Pimentel AC, Sichizya L, Lu Z, Piseaux-Aillon R, Kirchenbuechler D, Flozak AS, Gottardi CJ, Cuda CM, Perlman H, Jain M, Kamp DW, Budinger GR, Misharin AV. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur Respir J 55: 1900646, 2020. doi: 10.1183/13993003.00646-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Choi SM, Mo Y, Bang JY, Ko YG, Ahn YH, Kim HY, Koh J, Yim JJ, Kang HR. Classical monocyte-derived macrophages as therapeutic targets of umbilical cord mesenchymal stem cells: comparison of intratracheal and intravenous administration in a mouse model of pulmonary fibrosis. Respir Res 24: 68, 2023. doi: 10.1186/s12931-023-02357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ntolios P, Steiropoulos P, Karpathiou G, Anevlavis S, Karampitsakos T, Bouros E, Froudarakis ME, Bouros D, Tzouvelekis A. Cell therapy for idiopathic pulmonary fibrosis: rationale and progress to date. BioDrugs 34: 543–556, 2020. doi: 10.1007/s40259-020-00437-8. [DOI] [PubMed] [Google Scholar]
- 148. Tzouvelekis A, Toonkel R, Karampitsakos T, Medapalli K, Ninou I, Aidinis V, Bouros D, Glassberg MK. Mesenchymal stem cells for the treatment of idiopathic pulmonary fibrosis. Front Med (Lausanne) 5: 142, 2018. doi: 10.3389/fmed.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Raghu G, van den Blink B, Hamblin MJ, Brown AW, Golden JA, Ho LA, Wijsenbeek MS, Vasakova M, Pesci A, Antin-Ozerkis DE, Meyer KC, Kreuter M, Santin-Janin H, Mulder GJ, Bartholmai B, Gupta R, Richeldi L. Effect of recombinant human pentraxin 2 vs placebo on change in forced vital capacity in patients with idiopathic pulmonary fibrosis: a randomized clinical trial. JAMA 319: 2299–2307, 2018. doi: 10.1001/jama.2018.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sotiropoulou V, Karampitsakos T, Katsaras M, Papaioannou O, Tsiri P, Sampsonas F, Tzouvelekis A. What is new in the treatment of interstitial lung diseases. Pneumon 36: 1–9, 2023. doi: 10.18332/pne/161867. [DOI] [Google Scholar]
- 151.National Institutes of Health. ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT03832946.
- 152. Humphries DC, Mills R, Boz C, McHugh BJ, Hirani N, Rossi AG, Pedersen A, Schambye HT, Slack RJ, Leffler H, Nilsson UJ, Wang W, Sethi T, Mackinnon AC. Galectin-3 inhibitor GB0139 protects against acute lung injury by inhibiting neutrophil recruitment and activation. Front Pharmacol 13: 949264, 2022.doi: 10.3389/fphar.2022.949264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, Nilsson UJ, Leffler H, Sethi T, Tantawi S, Gravelle L, Slack RJ, Mills R, Karmakar U, Humphries D, Zetterberg F, Keeling L, Paul L, Molyneaux PL, Li F, Funston W, Forrest IA, Simpson AJ, Gibbons MA, Maher TM. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J 57: 2002559, 2021. doi: 10.1183/13993003.02559-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


