Figure 4.
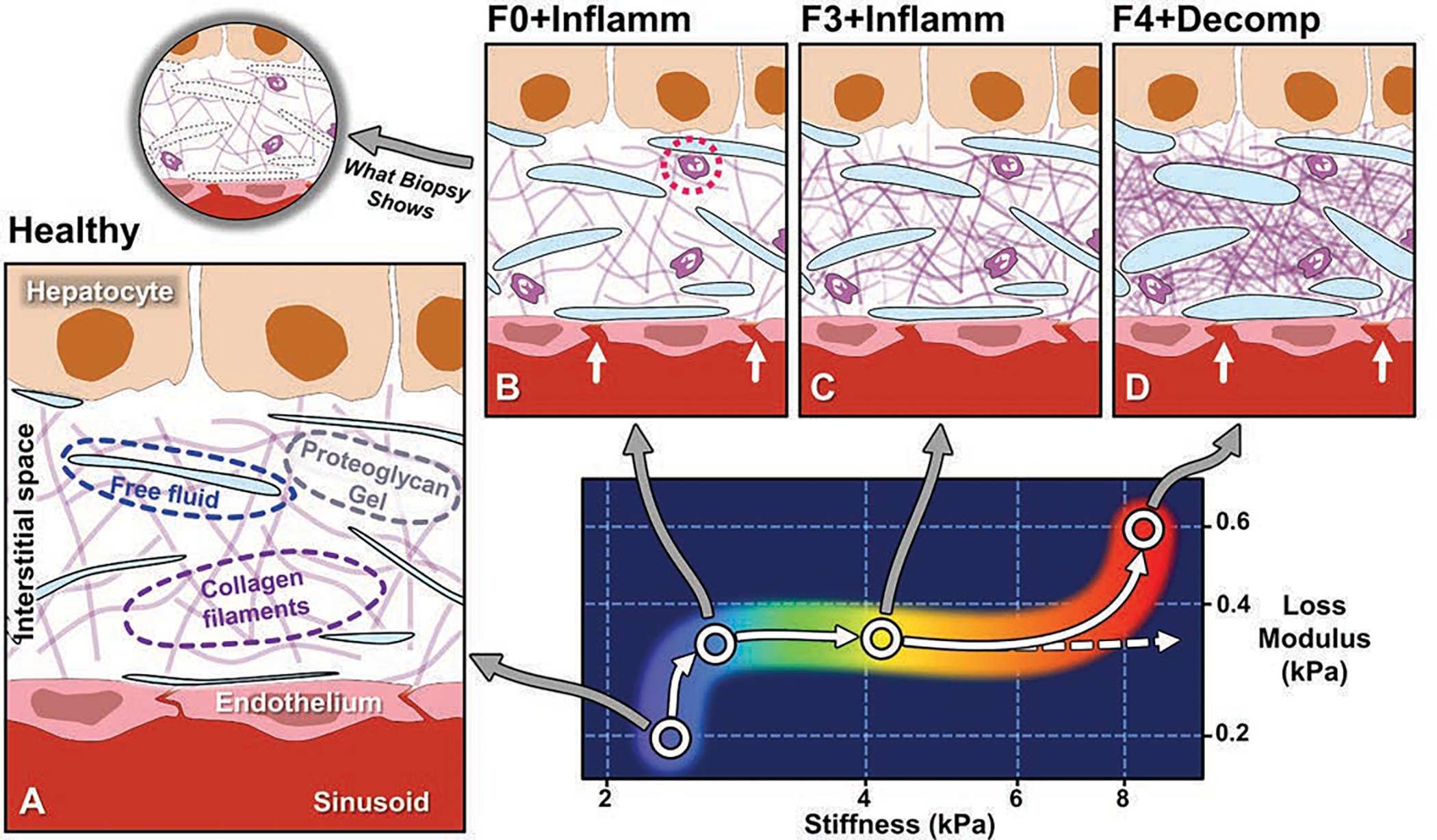
Conceptual illustration of emerging understanding of correlations between MRE-based quantitative biomarkers and spectrum of histopathology in chronic liver disease [67,79,81]. Panel A depicts hepatic interstitial space in healthy state. Interstitium is interposed between hepatic sinusoids and hepatocytes and consists of proteoglycan gel matrix with collagen filaments and other macromolecules. Normal interstitium also contains tiny clefts of free fluid, representing transudate from sinusoids, which is drained from interstitial space by lymphatics. These clefts of fluid are lost in standard process of biopsy tissue fixation and sectioning and therefore not generally visible at histologic review. Panel B shows inflammation, which is key mechanism in chronic liver disease and is detected at histopathology by presence of leukocytes in interstitium (dotted circle). Inflammation is also characterized by increased vascular permeability (arrows), resulting in increased interstitial fluid. Loss modulus is MRE-measured biomarker that is sensitive to fluid phase in tissue and rises with onset of inflammation. Increased loss modulus associated with inflammation also causes small increase in liver stiffness, independent of effect of fibrosis. In panel C, with activation of stellate cells, increased amounts of collagen accumulate in interstitial space. MRE-assessed liver stiffness largely reflects elastic properties of solid phase of tissue, and increases rapidly with fibrosis. Panel D depicts end-stage liver disease, in which accumulation of collagen leads to ever-increasing stiffness values (dashed arrow). Studies suggest that hepatic decompensation is marked by further elevation of loss modulus, with incrementally increased vascular permeability (arrows) and increased interstitial free fluid, potentially overloading lymphatic drainage of liver and resulting in ascites. MRE = MR elastography.
