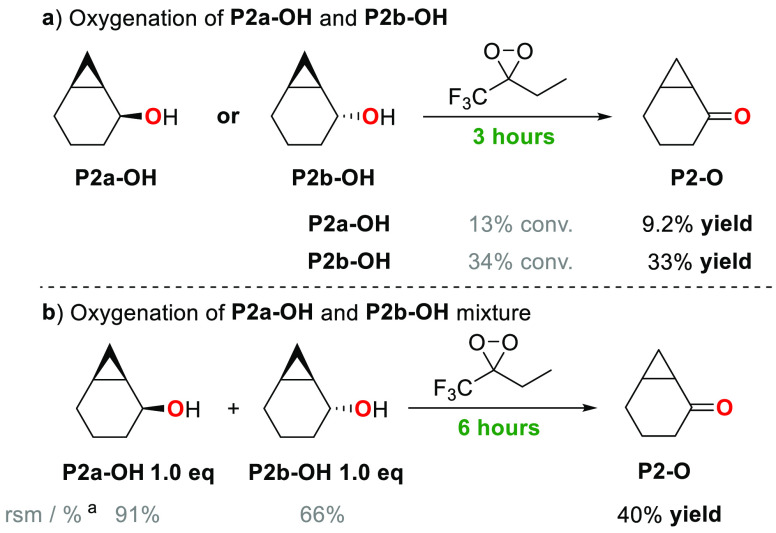
Scheme 5. Oxygenation of cis-Bicyclo[4.1.0]heptan-2-ol (P2a-OH) and trans-Bicyclo[4.1.0]heptan-2-ol (P2b-OH).
Conversion and product yields were determined by GC and averaged over two independent experiments. (a) Reaction conditions: P2a-OH or P2b-OH 1 equiv, oxone 1 equiv, NaHCO3 4 equiv, 1,1,1-trifluoro-2-butanone 0.2 equiv, HFIP/H2O (3:1), Bu4NHSO4 0.05 equiv, T = 0 °C, 3 h. (b) P2a-OH 1 equiv, P2b-OH 1 equiv, oxone 1 equiv, NaHCO3 4 equiv, 1,1,1-trifluoro-2-butanone 0.2 equiv, HFIP/H2O (3:1), Bu4NHSO4 0.05 equiv, T = 0 °C, 6 h. rsm: recovered starting material.