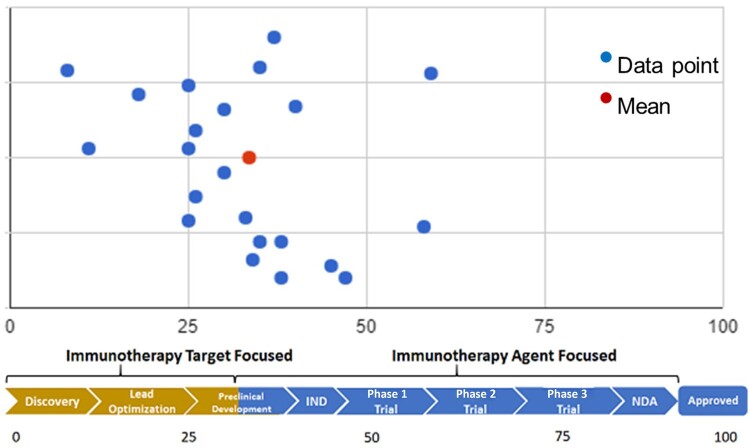
Figure 2.
Clinical Trials Task Force survey: response to the question of stage of target and/or agent on the clinical translation continuum. Responses to the Clinical Trials Task Force survey conducted in 2021 of Immuno-Oncology Translational Network members. More than 50% of Immuno-Oncology Translational Network investigators had an immuno-oncology target or agent already in the pipeline for eventual translation into clinical trials, with the mean being at the preclinical development stage. IND = investigational new drug; NDA = new drug application.