Summary
Background
Older studies uncovered an increased risk of cancer in patients with rheumatoid arthritis between 10% and 30% compared to the general population, with a lack of data concerning infrequent cancers. In recent year, major therapeutic breakthroughs might have affected this risk of cancer by mitigating disease activity or on the contrary by impairing antitumoral immune response. The objectives of this study are to compare cancer risk in patients with treated rheumatoid arthritis to the general population, in all treated patients and according to treatment exposure.
Methods
This is a nationwide population-based study within the French national claims database “Système National des Données de Santé” (SNDS) between January 1st 2010 and December 31st 2020, to estimate the age and sex-standardized incidence ratios of cancer (all sites and site specific) of treated rheumatoid arthritis patients, with the French population as reference (by use of the French Network of Population-Based Cancer Registries [FRANCIM]).
Findings
During the study period, 257,074 treated patients with rheumatoid arthritis contributed to a total of 2,098,238 person-years for the main analysis. The all-cancer risk was increased in rheumatoid arthritis patients, with a SIR (Standardized Incidence Ratio) of 1.20 (95% CI [1.17–1.23]). This risk was increased particularly for lung (SIR 1.41, 95% CI [1.36–1.46], bladder (SIR 2.38 95% CI [2.25–2.51]), cervix (SIR 1.80, 95% CI [1.62–2.01]), prostate (SIR 1.08, 95% CI [1.04, 1.13]) cancers, melanoma (SIR 1.37, 95% CI [1.29–1.46]), diffuse large B cell lymphoma (SIR 1.79, 95% CI [1.63–1.96], multiple myeloma (SIR 1.42, 95% CI [1.27–1.60]) and Hodgkin's lymphoma (SIR 2.73, 95% CI [2.31–3.23]). Some cancers were less frequent than in the general population such as pancreatic (SIR 0.90, 95% CI [0.83–0.97]) as well as breast and endometrial cancers (SIR 0.91, 95% CI [0.88–0.94] and SIR 0.77, 95% CI [0.71–0.84] respectively). Although we observed a modest but significant relative increase of all-cancer risk over-time in rheumatoid arthritis patients, there was a trend towards a decrease in risk of non-Hodgkin's lymphoma. Patients treated with rituximab were the patients displaying the highest risk of cancer.
Interpretation
Compared to the general population, treated rheumatoid arthritis patients were at greater risk of all-cancer and some site specific cancers, except for breast, pancreatic and endometrial cancers which were less frequent than in the general population.
Funding
This work was supported by unrestricted grants from the InCA (national institute against cancer) and AP-HP (Assistance Publique des Hôpitaux de Paris).
Keywords: Rheumatoid arthritis, Cancer, DMARD, bDMARDs, Lymphoma, Malignancy
Research in context.
Evidence before this study
We searched Pubmed on October 14th 2023 for observational studies assessing the risk of cancer in rheumatoid arthritis, using the terms “rheumatoid arthritis” and “cancer or malignancy” and “risk or incidence”. We found 21 studies of interest, including 4 limited to lymphoma. Of these, 10 included a comparison between rheumatoid arthritis patients and the general population. Among those 10 studies, 6 compared users of specific treatments to the general population (e.g., users of TNF inhibitor versus the general population) and only 4 compared all rheumatoid arthritis patients to the general population. Overall, the relative increase in all-cancer risk in rheumatoid arthritis patients was estimated between 10 and 30% compared to the general population, and between 60 and 80% for the risk of lymphoma. Regarding the association between treatment exposure and cancer incidence, some drugs such as TNF inhibitors have been closely studied and do not seem to be associated with an increased risk of cancer, except for some skin cancers. Rituximab is considered safe by previous studies and is used in clinical practice in patients with a high risk of cancer. Knowledge on the effect of other targeted treatments such as abatacept and anti-IL6 receptors have been less studied. In addition, JAK inhibitors are under continuous monitoring since the results of the ORAL surveillance study. Overall, given the recent therapeutic breakthroughs, we present an update on the risk of cancer in rheumatoid arthritis patients.
Added value of this study
This study reveals updated data regarding cancer risk in the disease burden of rheumatoid arthritis. We show that all-cancer risk remains higher by 20% in rheumatoid arthritis patients compared to the general population, with mostly tobacco linked cancers (lung, bladder, ears nose and throat), cervix and prostatic cancers, melanoma and hematological malignancies. Although all-cancer risk was relatively increased by 5% in the second half of our study period compared to the first half, the elevated risk of non-Hodgkin's lymphomas tended to decrease with time. Of note, breast, endometrial and pancreatic cancers were less frequent in rheumatoid arthritis patients than in the general population. In a secondary analysis, patients who were the most at risk of cancer were the ones currently exposed to rituximab and abatacept.
Implications of all the available evidence
Although we observed some encouraging results regarding non-Hodgkin's lymphoma, cancer risk remains high in patients with rheumatoid arthritis compared to the general population. To allow for early diagnosis of malignancy, physicians should be wary of this increase of risk, particularly in patients treated with abatacept or rituximab.
Introduction
Rheumatoid arthritis is the most common systemic auto-immune disease (0.5% of the population). It affects preferentially women in their sixties although it can be observed in both sex at any age. Rheumatoid arthritis patients are at higher all-cancer risk, with an excess relative risk of 28% compared to the general population as described by Mercer et al.1 a decade ago, however the estimation was imprecise for infrequent cancers due to insufficient follow-up. This risk is mostly carried by specific cancers such as lung cancer, Hodgkin's and non-Hodgkin's lymphomas along with skin cancers.2,3 The relationship between rheumatoid arthritis and lung cancer can easily be explained by tobacco use, a shared risk factor for both diseases, whereas lymphoma risk could be a consequence of chronic immune activation.4 Although tobacco exposure is beyond reach of therapeutic advances, its prevalence is decreasing in recent years in France5 which could lead to a decrease of smoking related cancer incidences.
Likewise, chronic immune activation in rheumatoid arthritis is also expected to decline in the era of biological and targeted synthetic Disease-Modifying Antirheumatic Drugs (b/tsDMARDs) which started in the early 2000s (full details on DMARD categories, commercialization dates and action mechanism are provided in Table S1). Studies exploring the evolution of the risk of cancer over time are lacking. By virtue of these major therapeutic breakthroughs to jugulate disease activity, one could hope for a decline of some cancers such as lymphomas in rheumatoid arthritis patients compared to older studies.
On the other hand, treatments with b/tsDMARDs might also increase the risk of cancer in rheumatoid arthritis patients by hampering anti-tumor immune response. There was long saga regarding the possible increased risk of cancer with anti-TNF after a first meta-analysis published in 2006,6 which was not confirmed later, except for non-melanoma skin cancers.7 Some questions persist with anti-TNF regarding melanoma and lymphoma risk. Lastly, recent signals suggest that rheumatoid arthritis treatments abatacept8 and tofacitinib9—could by themselves increase cancer risk. Overall, an update on cancer risk estimation in rheumatoid arthritis patients compared to the general population, in the era of modern biological treatment, using a large sample size with extended follow-up database is necessary.
We therefore conducted this study within the large French national claims database “Système National des Données de Santé” (SNDS), to compare the risk of cancer—all sites and site specific—of patients with rheumatoid arthritis to that of the general population and according to treatment exposure.
Methods
Study design
This is a nationwide population-based study conducted within the French national claims database between January 1st 2010 and December 31st 2020, to estimate the age and sex-standardized incidence ratios of cancer (all sites and site specific), with the French population as reference (by use of the French Network of Population-Based Cancer Registries [FRANCIM]10).
Data sources
In France, healthcare affiliation is free and universal. The general scheme is the most sizeable and covers around 90% of the French population, that is 60 million beneficiaries.11
Rheumatoid arthritis patients were identified in the general scheme of the French national healthcare database, the “Système National des Données de Santé” (SNDS). This database collects all primary care claims such as drug and devices reimbursement, type and date of in and out-patient visits as well as radiologic or biologic acts. It also collects data regarding diagnoses with International Classification of Diseases (ICD)-10 codes, either through “ALD” (long lasting disease) declaration or hospital discharge summaries.12
Reference population for cancer incidence was obtained through the FRANCIM register, which exhaustively collects cancer incidence in 23 French regional registries and estimates yearly cancer incidence in the whole French population. Cancer incidence is given by age, sex and calendar year for all cancers and by cancer sites, including hematological malignancies, excluding non-melanoma skin cancer. The FRANCIM register covers from 1990 to 2018 except for prostate cancer which ends in 2015. Individual data on patients to the register are not accessible.
Study population
Inclusion criteria
We identified patients ≥20 years-old, with an affiliation to the French health insurance general scheme, between January 1st 2010 and December 31st 2019 and affected by rheumatoid arthritis during the study period, without history of cancer. Rheumatoid arthritis was defined as at least one occurrence of ICD-10 M05 or M06 codes in hospitalization release or “ALD” declaration and the reimbursement of at least one DMARD. This latter condition was required to maximize the specificity of disease definition by fitting with European guidelines.13 Included subjects were required to have at least one year of affiliation to the health insurance general scheme prior to screening eligibility (i.e. first date with fulfillment of inclusion criteria). This period, defined as the minimal lookback period, allowed to differentiate prevalent from incident rheumatoid arthritis. It also allowed to exclude patients with an ICD-10 cancer code or cancer related health expenditure, including specific treatment or procedures, preceding their eligibility screening. Subjects were also required to have at least one year of affiliation following screening eligibility, to ensure they were regular consumers of the French health insurance.
Follow-up
The index date for prevalent rheumatoid arthritis patients was January 1st 2010. For incident rheumatoid arthritis patients, the index date was the date of first DMARD dispensation. Patients were followed-up until death or exit from the general insurance scheme or December 31st 2020. Although inclusions were limited to December 31st 2019, follow-up was extended until December 31st 2020 in order to ensure all patients were able to contribute at least one year.
Exposure definition
Cancer risk analysis in the overall treated rheumatoid arthritis population
In the primary analysis, exposure of interest was defined as having rheumatoid arthritis, with the risk period starting on January 1st 2010 for prevalent rheumatoid arthritis or at first dispensation of DMARD for incident rheumatoid arthritis. Thereafter, patients were considered as having rheumatoid arthritis until end of follow-up, regardless of patients’ continuation of DMARD.
Cancer risk analysis in rheumatoid arthritis patients according to current treatment exposure
In the secondary analyses, exposure was defined, among patients with rheumatoid arthritis, by the treatment received (categorized by molecular target). We distinguished csDMARDs (methotrexate, hydroxychloroquine, leflunomide, sulfasalazine), Tumor Necrosis Factor inhibitors (TNFi) (infliximab, adalimumab, golimumab, etanercept, certolizumab), rituximab, abatacept, Anti-Interleukine 6 receptors (anti-IL6R) (tocilizumab, sarilumab) and Janus Kinase inhibitors (JAKi) (tofacitinib and baracitinib). The risk period starts with a 90 days delay after first treatment dispensation (lag period), persists until treatment discontinuation (i.e. end of the period covered by the last treatment delivery (detailed in Table S1) with tolerated gaps of up to 90 days) and ends 180 days after treatment discontinuation (carry-over period). The risk period was thus not limited to incident treatment use. Therefore, a patient could contribute to multiple treatment exposure groups during the study period, and could contribute briefly (90 days) to two treatment exposure in case of treatment switch. Outcomes were attributed to the current exposure group(s) at the time of the event. Ultimately, simultaneous exposure to two b/tsDMARD was negligible.
Outcome
The outcome of interest was incident cancer identified according to Ajrouche et al. algorithm for SNDS14 with all-cancer risk defined as ICD-10 code C00 through C97 excluding C44 which stands for non-melanoma skin cancer (NMSC, not included in the FRANCIM registry), along with cancer specific drugs dispensation or procedures. Site specific cancer incidence was defined according to ICD-10 codes in the SNDS and ICD for Oncology 3 in FRANCIM.
Ethics and legislation
This observational study used existing claims data (SNDS) and yearly aggregated data by sex and age categories from the FRANCIM register. The study protocol has been approved by the French « Commission Nationale Informatique et Libertés » (CNIL) (authorization n° MLD/MFI/AR2012850) governing data access and privacy laws. As this study involved anonymized data, informed consent from patients was not required (see Social Security Code, article L161–28-1). Moreover, according to the French legislation, process to access such administrative database did not require submission to an ethics committee.
Patient involvement
No patient was involved in the study design, data analysis nor interpretation.
Statistical analysis
Cancer risk in rheumatoid arthritis compared to the general population
We compared the incidence of cancer (all site excluding NMSC and site specific) in patients with rheumatoid arthritis to that of the general population in France by calculating the Standardized Incidence Ratio (SIR) of cancer and their 95% confidence intervals.
For privacy concerns, all analyses were conducted on aggregated data. Individual patient records were aggregated by a certified statistician in summary tables comprising of number of all-cancer and site specific cancer cases and number of person-time by sex, calendar year and age category in each calendar year stratum given by 5 years’ interval from 20 to over 85 years old. For secondary analyses, aggregation was further split by treatment exposure.
Cancer incidence was considered to follow a Poisson distribution. For each age, sex and calendar year group, the expected number of cancers in the rheumatoid arthritis population based on cancer incidence rates provided by FRANCIM were estimated. SIR were then tested to 1 (i.e. absence of difference) by a Poisson regression model with the number of observed cases as the dependent variable and the log of expected cases as offset. Cancer risk was estimated in the whole rheumatoid arthritis population for the primary analysis and according to treatment exposure for secondary analyses. In case of overdispersion, negative binomial regression was used instead of Poisson regression models. Overdispersion was observed in the main analysis for all-cancer and multiple myeloma.
Subgroup analysis
We applied the same approach in the following pre-specified subgroups: age <65 and ≥65 years-old as well as male and female, calendar year before 2016 (middle of the study period) or including 2016 and after. Between-group incidence rate ratios (IRR), which is the between-group ratio of SIR, were estimated for each subgroup analysis.
Sensitivity analyses
We repeated the primary and secondary analysis with a fixed lag period of 90 days and varying carry-over periods of 24 and 60 months, the latter being very close from an “ever treated analysis”. Another analysis was carried out without the age categories of 80 years-old and older to address concerns of missing cancers in elderly patients in the SNDS, as a suspected cancer diagnosis might not be fully investigated in this population if no treatment is ultimately warranted. Therefore, since cancers are detected through hospitalization discharges summaries, “ALD” or specific treatment or procedures in the SNDS, estimation of cancer incidence in elderly rheumatoid arthritis patients could be underestimated. We repeated the main analysis excluding the years 2019–2020 as FRANCIM's projection terminate in 2018, and conducted an analysis restricted to incident rheumatoid arthritis only. To explore reverse causation, we conducted an analysis excluding cancers occurring within the first two years after rheumatoid arthritis diagnosis. Finally, we performed the main analysis on all patients with an ICD-10 code for rheumatoid arthritis within the SNDS regardless of reimbursement of a DMARD.
Role of the funding source
This study was supported by unrestricted grants from the nation institute against cancer and the Paris public hospital association. The funding sources did not participate in the design, data curation, data analysis nor writing of the manuscript.
Results
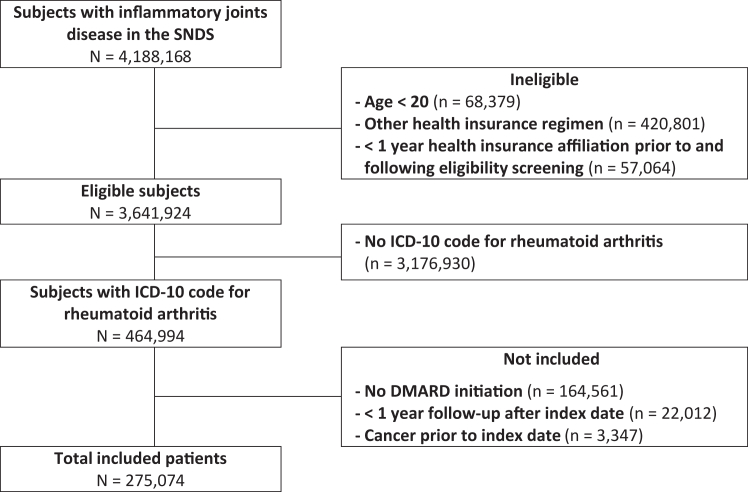
During the study period, we included 257,074 patients with rheumatoid arthritis, aged ≥20 years, and treated with DMARDs who contributed to a total of 2,098,238 person-years for the main analysis, with a median follow-up of 8.7 years [Interquartile Range (IQR) 4.8–11.9]. A flow chart is presented in Fig. 1. Median age at inclusion was 58.3 [IQR 48.0–68.7] and 189,335 (73.6%) of patients were women (Table 1).
Fig. 1.
Flow Chart. SNDS: système national des données de santé, DMARD: disease-modifying anti-rheumatic drug, ICD: international classification of diseases.
Table 1.
Rheumatoid arthitis patients’ characteristics at inclusion.
| Male | Female | |
|---|---|---|
| Population description | ||
| Subjects (n) | 67,739 | 189,335 |
| Person-years | 52,6972 | 1,571,266 |
| Age at inclusion (median [IQR]) | 59.4 [49.9–68.8] | 57.80 [47.3–68.7] |
| Follow-up duration (median, IQR) | 7.78 [4.28–11.70] | 9.05 [5.06–11.95] |
| Cancers per subject (n, %) | ||
| 0 | 58,759 (86.7%) | 175,791 (92.8%) |
| 1 | 7606 (11.2%) | 12,076 (6.4%) |
| ≥2 | 1374 (2.1%) | 11,468 (0.8%) |
| Rheumatoid arthritis status | ||
| Prevalent | 28,114 (41.5%) | 88,966 (47.0%) |
| Incident | 39,625 (58.5%) | 100,369 (53.0%) |
| Treatment exposure | ||
| csDMARD alone | ||
| Subjects exposed (N) | 61,498 | 17,4767 |
| Exposure, years (median, IQR) | 5.42 [2.64–9.63] | 6.06 [2.87–10.65] |
| Person-years | 271,318 | 828,003 |
| TNFi | ||
| Subjects exposed (N) | 17,764 | 48,233 |
| Exposure, years (median, IQR) | 4.95 [2.04–9.24] | 4.50 [1.86–8.61] |
| Person-years | 86,800 | 222,834 |
| Anti-IL6R | ||
| Subjects exposed (N) | 3279 | 11,706 |
| Exposure, years (median, IQR) | 2.02 [0.96–4.30] | 2.15 [0.97–4.66] |
| Person-years | 34,057 | 8883 |
| Rituximab | ||
| Subjects exposed (N) | 3219 | 10,179 |
| Exposure, years (median, IQR) | 1.79 [0.86–3.64] | 1.95 [0.87–4.05] |
| Person-years | 8396 | 28,521 |
| Abatacept | ||
| Subjects exposed (N) | 3018 | 11,514 |
| Exposure, years (median, IQR) | 1.85 [0.92–3.81] | 1.84 [0.91–3.97] |
| Person-years | 7386 | 29,247 |
| JAKi | ||
| Subjects exposed (N) | 1423 | 5639 |
| Exposure, years (median, IQR) | 1.48 [0.87–2.18] | 1.42 [0.82–2.13] |
| Person-years | 1701 | 6639 |
csDMARD: conventional disease-modifying antirheumatic drug, TNFi: Tumor necrosis factor inhibitor, JAKi: Janus Kinase inhibitor.
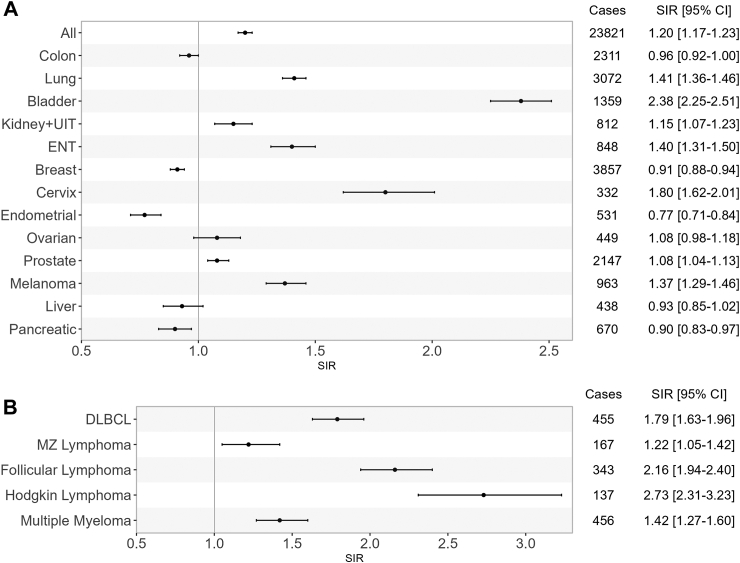
A total of 23,821 all-cancer cases were observed with most frequent being breast cancer (n = 3857), lung cancer (n = 3072), colon cancer (n = 2311) and prostatic cancer (n = 2147). Incidence rates by cancer site according to population, age category and sex are shown in Figure S1 and Table S2.
Cancer risk in patients with rheumatoid arthritis
Compared to the French general population, all-cancer risk was increased in rheumatoid arthritis patients (Fig. 2A) with a SIR of 1.20 (95% CI [1.17–1.23]). We observed an increased risk of lung (SIR 1.41, 95% CI [1.36–1.46]), bladder (SIR 2.38 95% CI [2.25–2.51]), cervix (SIR 1.80, 95% CI [1.62–2.01]), prostate (SIR 1.08, 95% CI [1.04, 1.13]) cancer and melanoma (SIR 1.37, 95% CI [1.29–1.46]). Hematological cancers were also more frequent in rheumatoid arthritis patients (Fig. 2B), including diffuse large B cell lymphoma (SIR 1.79, 95% CI [1.63–1.96]), follicular lymphoma (SIR 2.16, CI 95% [1.94–2.40]) and Hodgkin's lymphoma (SIR 2.73, 95% CI [2.31–3.23]). Finally, multiple myeloma (SIR 1.42, 95% CI [1.27–1.60]) were also more frequent in patients with rheumatoid arthritis than in the general population. On the contrary, pancreatic cancer was less frequent than in the general population (SIR 0.90, 95% CI [0.83–0.97]) as well as some female specific cancers such as breast and endometrial cancers (SIR 0.91, 95% CI [0.88–0.94] and SIR 0.77, 95% CI [0.71–0.84], respectively). All absolute incident rates are presented in Table S2.
Fig. 2.
Standardized incidence ratios (SIR) of cancers in rheumatoid arthritis patients compared to general population. Panel A: solid cancers, panel B: hematological cancers, All: all cancers excluding non-melanoma skin cancer, ENT: ears, nose, throat, DLBCL: diffuse large B cell lymphoma, MZ: marginal zone Cases: Observed cancer cases, SIR: standardized incidence ratio, CI: confidence interval.
Subgroup analysis
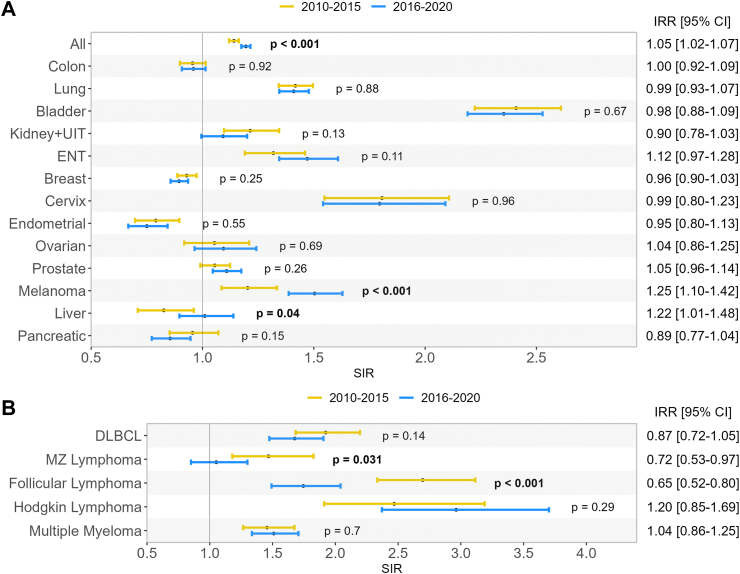
Trends in risk of cancer over time
The increased risk of cancer in rheumatoid arthritis patients as compared to the general population was significantly higher during the 2016–2020 (SIR 1.19 95% CI [1.17–1.22]) than during the 2010–2015 period (SIR 1.14 95% CI [1.12–1.16]), although relative difference was modest with an incidence rate ratio (IRR) of 1.05 95% CI [1.02–1.07]. This increase in risk was partly driven by a significant increased risk of melanoma and liver cancer (Fig. 3A) among rheumatoid arthritis patients compared to the general population in the 2016–2020 period compared to 2010–2015 period. Of note, the increased risk of some non-Hodgkin's lymphoma such as marginal zone lymphoma and follicular lymphoma were significantly lower in the 2016–2020 compared to the 2010–2015 period (Fig. 3B) (IRR 0.72, 95% CI [0.53–0.97] and IRR 0.65, 95% CI [0.52–0.80] respectively).
Fig. 3.
Standardized incidence ratios (SIR) of cancers in rheumatoid arthritis patients compared to general population according to calendar period. Panel A: solid cancers, panel B: hematological cancers, All: all cancers excluding non-melanoma skin cancer, ENT: ears, nose, throat, DLBCL: diffuse large B cell lymphoma, MZ: marginal zone, IRR: incidence rate ratio (male versus female), CI: confidence interval, SIR: standardized incidence ratio, p-values represent the between-group difference in incidence rate ratio.
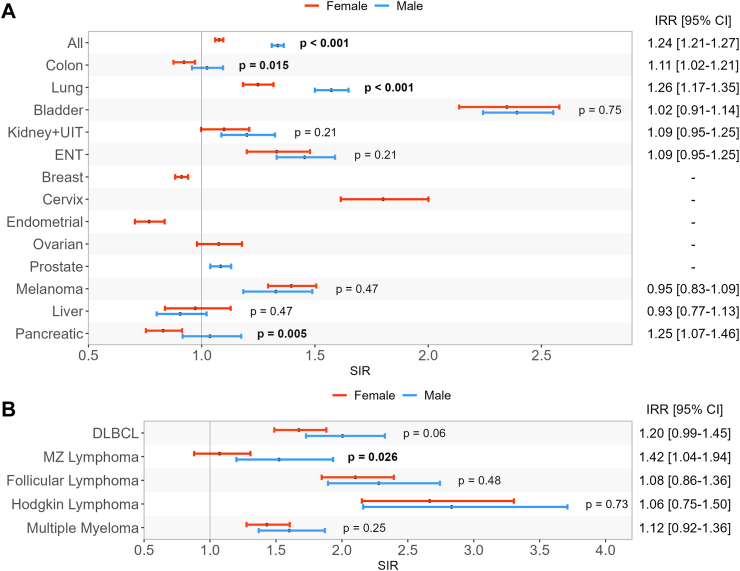
Sex-differences in cancer risk
The SIR for all-cancer in men was 1.34 (95% CI 1.31–1.36] and 1.08 (95% CI 1.06–1.10) in women. The estimated incidence rate ratio (IRR) between men and women was of 1.24 (95% CI [1.21–1.27]) (Fig. 4A). This difference was mostly driven by higher risk of lung cancer, colon cancer and pancreatic cancer in men than in women with rheumatoid arthritis, compared to the general population (Fig. 4A). Moreover, as stated above, some frequent sex-specific cancers in women with rheumatoid arthritis tend to be less frequent than in the general population such as breast and endometrial cancer contrarily to prostate cancer risk, which was more frequent in patients with rheumatoid arthritis compared to the general population.
Fig. 4.
Standardized incidence ratios (SIR) of cancers in rheumatoid arthritis patients compared to general population according to sex. Panel A: solid cancers, panel B: hematological cancers, All: all cancers excluding non-melanoma skin cancer, ENT: ears, nose, throat, DLBCL: diffuse large B cell lymphoma, MZ: marginal zone, IRR: between-group incidence rate ratio (2016–2020 versus 2010–2015), CI: confidence interval, IRR:incidence rate ratio, SIR: standardized incidence ratio, p-values represent the between-group difference in incidence rate ratio.
Cancer risk in rheumatoid arthritis patients according to treatment exposure
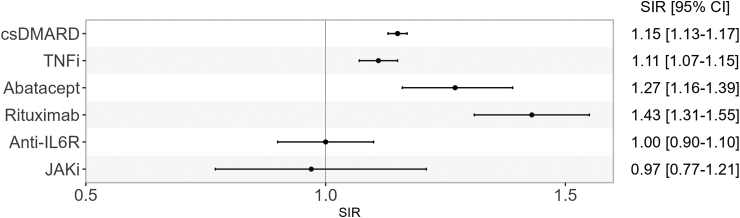
In analyses according to treatment exposure (Table 2, Table 3), we observed that, compared to the general population, all-cancer risk was increased in patients while exposed to csDMARD (SIR 1.15, 95% CI [1.13–1.17]), TNFi (SIR 1.11, 95% CI [1.07–1.15]), abatacept (SIR 1.27, 95% CI [1.16–1.39]), and rituximab exposure, the latter carrying the greatest observed risk (SIR 1.43, 95% CI [1.31–1.55]) (Fig. 5). This increased risk was not observed in patients exposed to anti-IL6R (SIR 1.00, 95% CI [0.90–1.10]). Although we did not observe any difference in cancer risk compared to the general population in patients while exposed to JAKi (SIR 0.97, 95% CI [0.77–1.21]), it should be noted that number of subjects and person-years for JAKi exposure was relatively small (n = 7,062, person-years = 8340). For this reason, site-specific cancers under JAKi exposure are not presented. Although Hodgkin's lymphoma and multiple myeloma occurred more frequently in rheumatoid arthritis patients across all treatment categories than in the general population, the association's magnitude was considerably higher for patients under rituximab for Hodgkin's lymphoma (SIR 6.44, 95% CI [2.89–14.39] and for multiple myeloma SIR 8.81 95% CI [6.61–11.74]).
Table 2.
Standardized incidence ratio of solid cancer by treatment compared to the general population.
| Cancers | csDMARD | TNFi | Abatacept | Rituximab | Anti-IL6R |
|---|---|---|---|---|---|
| Alla | 1.15 [1.13–1.17] | 1.11 [1.07–1.15] | 1.27 [1.16–1.39] | 1.43 [1.31–1.55] | 1.00 [0.90–1.10] |
| Colon | 0.95 [0.90–1.00] | 0.96 [0.85–1.08] | 1.06 [0.79–1.43] | 1.15 [0.87–1.53] | 0.81 [0.58–1.13] |
| Lung | 1.42 [1.35–1.48] | 1.41 [1.28–1.55] | 2.10 [1.69–2.59] | 1.68 [1.33–2.12] | 1.15 [0.87–1.53] |
| Bladder | 2.37 [2.21–2.54] | 2.40 [2.05–2.82] | 2.37 [1.54–3.64] | 2.96 [2.04–4.29] | 2.87 [1.94–4.25] |
| Kidney + UIT | 1.12 [1.03–1.23] | 1.16 [0.95–1.40] | 1.11 [0.65–1.87] | 0.70 [0.36–1.34] | 1.06 [0.63–1.79] |
| ENT | 1.36 [1.24–1.48] | 1.37 [1.15–1.64] | 1.26 [0.75–2.13] | 1.71 [1.10–2.66] | 1.31 [0.80–2.14] |
| Breast | 0.91 [0.88–0.95] | 0.87 [0.80–0.95] | 0.84 [0.67–1.06] | 0.67 [0.52–0.87] | 0.69 [0.55–0.88] |
| Cervix | 1.93 [1.68–2.21] | 1.44 [1.06–1.95] | 1.32 [0.54–3.19] | 1.35 [0.56–3.26] | 2.28 [1.22–4.26] |
| Endometrial | 0.80 [0.72–0.89] | 0.76 [0.60–0.97] | 0.50 [0.24–1.05] | 0.82 [0.45–1.48] | 0.82 [0.46–1.45] |
| Ovarian | 1.01 [0.89–1.14] | 1.47 [1.18–1.84] | 1.56 [0.90–2.69] | 0.87 [0.41–1.85] | 1.26 [0.69–2.28] |
| Prostate | 1.15 [1.09–1.21] | 0.78 [0.68–0.90] | 1.18 [0.85–1.63] | 0.93 [0.67–1.31] | 0.86 [0.59–1.25] |
| Melanoma | 1.45 [1.34–1.58] | 1.13 [0.93–1.35] | 1.49 [0.96–2.32] | 1.35 [0.85–2.15] | 1.23 [0.77–1.95] |
| Liver | 0.69 [0.60–0.79] | 1.07 [0.83–1.37] | 1.50 [0.85–2.65] | 0.83 [0.40–1.75] | 0.49 [0.18–1.31] |
| Pancreatic | 0.79 [0.71–0.88] | 0.98 [0.79–1.21] | 1.04 [0.62–1.76] | 0.92 [0.52–1.62] | 0.59 [0.29–1.18] |
All cancer except non melanoma skin cancer; ENT: ear, nose throat, UIT: upper urinary tract.
Table 3.
Standardized incidence ratio of hematological cancer by treatment compared to the general population.
| Cancers | csDMARD | TNFi | Abatacept | Rituximab | Anti-IL6R |
|---|---|---|---|---|---|
| DLBCL | 1.89 [1.68–2.12] | 1.54 [1.15–2.05] | 3.16 [1.87–5.35] | 2.27 [1.22–4.23] | 1.32 [0.59–2.94] |
| MZ Lymphoma | 1.10 [0.90–1.36] | 1.57 [1.07–2.31] | 1.99 [0.82–4.79] | 2.87 [1.36–6.03] | 0.39 [0.05–2.77] |
| Follicular Lymphoma | 2.28 [1.99–2.61] | 2.14 [1.61–2.86] | 0.98 [0.31–3.04] | 2.61 [1.30–5.24] | 0.31 [0.04–2.19] |
| Hodgkin Lymphoma | 2.62 [2.09–3.29] | 2.88 [1.92–4.30] | 1.10 [0.15–7.88] | 6.44 [2.89–14.39] | 1.80 [0.45–7.25] |
| Multiple Myeloma | 1.20 [1.06–1.37] | 1.10 [0.80–1.50] | 2.22 [1.26–3.92] | 8.81 [6.61–11.74] | 2.21 [1.25–3.90] |
DLBCL: Diffuse Large B Cell Lymphoma, MZ: Marginal Zone.
Fig. 5.
All cancer risk (except non-melanoma skin cancer) in rheumatoid arthritis patients according to treatment exposure compared to the general population. csDMARD: conventional disease-modifying antirheumatic drug, TNFi: Tumor necrosis factor inhibitor, JAKi: Janus Kinase inhibitor, SIR: standardized incidence ratio, CI: confidence interval.
Sensitivity analyses
When restricting the main analysis to incident rheumatoid arthritis patients, result for all-cancer risk in rheumatoid arthritis compared to the general population was unchanged, and trends according to cancer site were similar (Figure S3). Excluding patients older than 80 years-old did not impact results significantly although overall, cancer risk tends to be higher when excluding patients older than 80 years-old, in particular for bladder cancer (Figure S4). Truncating the years 2019–2020 did not impact results of the main analysis (Figure S5). Of note, applying a lag period of 2 years after the diagnosis of rheumatoid arthritis tended to decrease cancer risk (Figure S6) in rheumatoid arthritis patients, as expected. When extended the main analysis to all patients with an ICD-10 code of rheumatoid arthritis regardless of DMARD reimbursement, overall and site-specific cancers risk tended to be less important than in our main analysis (Figure S7). Finally, analyses with increasing carry-over periods show similar trends with the main analysis (Figure S8).
Discussion
This large observational study, to our knowledge, showcases the longest average follow-up regarding cancer risks in patients with rheumatoid arthritis. It confirms an increased risk of all-cancer in rheumatoid arthritis patients compared to the general population, with a relative excess of risk of 20%. This excess of risk was essentially carried by men who displayed a relative excess of all-cancer risk of 34%, whereas women demonstrated a comparatively smaller increase in relative risk, at 8%, compared to the general population. Some site specific cancers were overrepresented in rheumatoid arthritis such as lung, bladder, kidney and upper urinary tract, melanoma, ovarian, cervix and prostate cancer as well as diffuse large B cell lymphoma, follicular, Hodgkin's and marginal zone lymphoma, as well as multiple myeloma. Interestingly, we observed a decreased risk of breast, endometrial and pancreatic cancer in rheumatoid arthritis patients compared to the general population. Patients currently exposed to rituximab or abatacept faced the highest cancer risk, specifically exhibiting a markedly higher incidence of multiple myeloma and Hodgkin's lymphoma in the case of rituximab, and of lung cancer in the case of abatacept compared to the general population.
The all-cancer relative excess of risk estimated in our study was higher to Wolfe et al.‘s15 estimation in a study published in 2007, involving nearly 14,000 subjects, with a SIR of 1.0 [95% CI 1.0–1.1]. However, our estimation was similar to Dreyer's et al.‘s3 Danish cohort of 10,000 subjects spanning from 2000 to 2008, which reported a SIR of 1.25 [95% CI 1.05–1.48] for all-cancer in rheumatoid arthritis patients not treated by TNFi versus the general population, and of 1.27 [95% CI 1.08–1.49] in patients treated by TNFi versus the general population. Our estimation is also similar to Mercer et al.‘s1 study which extended from 2002 to 2009 in the UK, and included 13,000 subjects. They identified a SIR of 1.28 [95% CI 1.10–1.48] for all-cancer among rheumatoid arthritis patients not treated by biologics. However, it should be mentioned the definition of all-cancer was broader in Dreyer's study than in Mercer's and ours. The former study encompassed non-melanoma skin cancers, which are more prevalent in rheumatoid arthritis patients than in the general population and thus significantly contribute to the overall cancer burden in rheumatoid arthritis patients.
Behind this slight increase in all-cancer risk lies a more complex relationship between rheumatoid arthritis and site-specific cancers. To begin, we observed a clear increase in smoking related cancers, namely lung, bladder, kidney and upper urinary tract, and ears, nose, throat (ENT) and cervix cancers. The relationship between tobacco use and risk of rheumatoid arthritis is well established, with a higher risk of rheumatoid arthritis in men than women smokers,16 possibly because of more intense tobacco exposition in men smokers than in women. This is partly concordant with our results as men with rheumatoid arthritis were more at risk of lung cancer than women with rheumatoid arthritis, which hints towards higher tobacco consumption in men than women with rheumatoid arthritis.
Secondly, the SIR of cancer in women was of 1.08 compared to 1.34 in men. In particular, endometrial and breast cancer were less frequent than in the general population, specifically in patients over 65. We further explored if the reason behind this risk reduction could be an underdiagnosis of cancers in the very elderly within the SNDS, however this decrease in risk was also found in patients aged under 80. Wadström et al. showed in a nationwide registry based case–control study that patients with rheumatoid arthritis were at lower risk of breast cancer than controls (HR 0.80 [0.68–0.93]), regardless of whether rheumatoid arthritis diagnosis was made prior or following breast cancer diagnosis.17 Interestingly, a similar risk profile can be observed in coeliac disease with a reduced risk of breast, ovarian and endometrial cancer.18 These results suggest that some hormonal factors may act both as protective factors of some cancers and as a risk factor of auto-immune diseases. For instance, in a prospective cohort study, we observed that early menopause and younger age at first pregnancy were associated with an increased risk of rheumatoid arthritis.19 Given that both these factors are associated with a reduced risk of breast cancer, endogenous hormonal exposure could potentially contribute to the lower incidence of breast cancer in patients with rheumatoid arthritis as compared to the general population.
Cervix cancers on the other hand were noticeably more frequent in rheumatoid arthritis. Given that almost all cases of cervix cancers are secondary to the human papillomavirus (HPV), this result suggests that treated rheumatoid arthritis patients, because of their disease and treatment-related impaired immunity, are more susceptible to HPV-related cancers. This hypothesis if reinforced by the increased risk of ENT cancers in rheumatoid arthritis which risk is also, to a lesser extent, increased with HPV infection. Moreover, cervix cancer might appear more frequent in rheumatoid arthritis because of ascertainment bias, since patients with chronic illnesses are likely to use health care more frequently than otherwise healthy individuals and thus might benefit from better screening than healthy subjects. However, this effect was not observed for breast cancer, which risk was lower in patients with rheumatoid arthritis than in the general population, pleading that ascertainment bias is not the only factor driving the increased risk of cervix cancer.
Surprisingly, pancreatic cancer was also less frequent in rheumatoid arthritis patients than in the general population. Despite our results being consistent across subgroup and sensitivity analyses, they differ from a previous US study based on multiple healthcare providers’ electronic records, spanning from 1999 to 2019.20 This study estimated an increased risk of pancreatic cancers among rheumatoid arthritis patients however this study did not use general population comparators but patients from a healthcare facility. Conversely, an exploratory case–control study estimated a lower risk of pancreatic cancer in rheumatoid arthritis patients and suggested the existence of opposite environmental or genetic factors between pancreatic cancer and auto-immune disease risk.21
The increased risk of lymphoma, either Hodgkin's lymphoma or non-Hodgkin's lymphoma is well known in rheumatoid arthritis patients and our results are in line with the literature.4 However, an important and encouraging result is the decrease in risk of some non-Hodgkin's lymphoma in the 2016–2020 period compared to 2010–2015, confirming a previous study carried by the ARTIS group.22 As chronic inflammation is one of the main drivers of lymphomagenesis in rheumatoid arthritis,23 we hypothesize this decrease might reflect the major therapeutic advances over the past two decades, which led to great improvement in controlling chronic inflammation. Conversely, it is interesting to note that the risk of Hodgkin's lymphoma did not decrease between study periods. Risk of Hodgkin's lymphoma, which is frequently associated with Epstein–Barr Virus (EBV), could be favored by immunosuppressive treatments. Another noteworthy result was the increased risk of multiple myeloma in patients with rheumatoid arthritis than in the general population. The relationship between rheumatoid arthritis and multiple myeloma has been scarcely studied with conflicting results, some studies finding an increased risk while others not,24,25 although compared to our study, sample size was limited.
Some cancers which are not associated with disease activity nor tobacco, such as prostatic cancer or melanoma, were more frequent in rheumatoid arthritis patients compared to general population with similar trends across treatment exposure. This suggests either common risk factors between these cancers and rheumatoid arthritis, which is not currently identified, or, more likely, an effect of immunosuppressive treatments on the risk of these cancers As mentioned previously, this is well known for cervix cancer,26 and has been described in other inflammatory diseases for melanoma.27
In the analysis of cancer according to treatment exposure, it is striking to observe that the highest risk of cancer was observed in patients treated by rituximab and abatacept. This contrasts with a similar increase of risk in patients exposed to csDMARDs, TNFi, or even a risk similar to that of the general population in rheumatoid arthritis patients treated with anti-IL6R. We remind that this study is descriptive and we do not infer a causal relationship between treatment exposure and cancer risk. These differences probably underline first and foremost various patient profiles according to treatment exposure.
Rituximab is the oldest biologic and is considered to be safe regarding the risk of cancer, being the bDMARD of choice in patients with a high risk of cancer, which might clearly induce an indication bias for this biologic. In the ARTIS registry, where indication bias might have been taken into account with adjustment for comorbidities, no increased risk of cancer was found in 4123 patients with rheumatoid patients treated with Rituximab with a median follow-up of more than 5 years.28 Anti-citrullinated protein antibody (ACPA) or rheumatoid factor positivity might also bias the association between hematologic cancer and rituximab. Seropositivity is associated both with tobacco smoking and with non-Hodgkin's lymphoma in rheumatoid arthritis.29 Given that, in clinical practice, rituximab is preferentially used in ACPA positive patients because of better efficacy,30 there might be an indication bias towards rituximab prescription in patients with the highest risk of cancer. This illustrates the intricate relationship between patient profile, treatment exposure and cancer risk. Likewise, the subtype of cancer the most linked to rituximab is multiple myeloma (SIR = 8.81 [6.61–11.74]), possibly because patients with monoclonal components of unknown significance, that is a pre-multiple myeloma stage, were more prone to receive rituximab. Of note, the importance of B cells for the efficacy of immune check points in cancer has recently been highlighted31 and, overall, physicians should be wary of hematological and tobacco-linked cancers in patients treated with rituximab. We issue a similar warning concerning abatacept exposure, particularly for melanoma risk which was highest in patients exposed to abatacept in our study. Two studies showed an increased risk of melanoma in patients treated with abatacept,28,32 and, although these results are not consistent across studies,8,33 our results warrant caution. Last, no conclusion can be drawn on tofacitinib and JAK inhibitors in general since follow-up was limited.
Our study presents several strengths, the main one being its representativeness, as it sought to include all rheumatoid arthritis patients from the French nationwide comprehensive database. Also, this database spans from more than a decade and allowed for a duration of follow-up extending far beyond previous studies. Its large sample size also allowed to study some cancers with a very low incidence rate such as Hodgkin's lymphoma or individual non-Hodgkin's lymphoma, unlike previous studies which were underpowered. Finally, as our inclusion criteria were broad and all rheumatoid arthritis patients treated at least once with a DMARD were included, we believe our results are generalizable to most clinical situations, and might help clinicians to adequately screen and monitor rheumatoid arthritis patients regarding their actual risk of cancer.
This study's main limitation is that the only factors accounted for were age, sex and calendar year, by design. However, the main strength is its representativeness since involving all treated rheumatoid arthritis cases from the nationwide healthcare database covering almost all French population. The aim of this study was primarily to depict a picture of cancer risk in rheumatoid arthritis patients rather than explore a causal link between rheumatoid arthritis, its treatments and cancer development. The reported associations between treatment exposure and cancer risk are solely descriptive and are designed to be practical aids for physician to assess cancer risk for each patient.
Another limitation is the absence of validated algorithm within the SNDS to identify patients with rheumatoid arthritis. This could raise concerns regarding misclassification. However, we used the same algorithm to identify rheumatoid arthritis patients as the one used in a recent study estimating the prevalence of rheumatoid arthritis in France,34 which found an estimated prevalence of rheumatoid arthritis very similar to results from an ad hoc and well conducted epidemiological study.35 We however restricted our analysis to all subjects who received at least one DMARD. We therefore repeated our main analysis including all patients with an ICD-10 code of rheumatoid arthritis regardless of DMARD reimbursement, and observed a risk of cancer in the rheumatoid arthritis population closer to the risk in the general population. This results could be explained by the fact that untreated patients might either not effectively have rheumatoid arthritis, or present with milder cases. In either case, restricting the analysis to patients receiving at least one DMARD is in our opinion more reflective of clinical practice13 and increases specificity.
Moreover, although we acknowledged that smoking status is a strong driver for cancer risk, we were not able to adjust for this factor or to compare cancer risk differentially among smokers and non-smokers, as this information is lacking for the general population (FRANCIM data). This was, however, not one of the initial objectives of our study, as we wished to estimate cancer risk in all rheumatoid arthritis patients, to increase awareness of physician on the necessity/usefulness of an appropriate monitoring. It is possible that the magnitude of the increased SIR for smoking-related cancers could be almost entirely attributed to smoking, and this point is important to be addressed in further studies.
Moreover, although we acknowledge smoking status is a strong driver for cancer risk, we were not able to adjust for this factor or to compare cancer risk differentially among smokers and non-smokers, as this information is lacking for the general population (FRANCIM data). This was, however, not one of the initial objectives of our study, as we wished to estimate cancer risk in all rheumatoid arthritis patients, to increase awareness of physician on the necessity/usefulness of an appropriate monitoring. It is possible that the magnitude of the increased SIR for smoking-related cancers could be almost entirely attributed to smoking, and this point is important to be addressed in further studies. Another limit was that the FRANCIM cancer registry coverage period did not include the calendar years 2019 and 2020 used in the primary analysis. However, a sensitivity analysis excluding the calendar years 2019–2020 has shown similar results.
Finally, as is the case with most medico-administrative database, individual medical records data are lacking and we were note able to further decline our results depending on serological status or the presence of bone erosion. Future studies should address these limitations, and should in particular better outline risk factors associated with cancer risk in rheumatoid arthritis patients. The assessment of factors, either environmental or constitutional, associated either with higher or lower risk of cancer, is pivotal towards promoting primary prevention and targeted screening. Further investigations should also explore the correlation between rituximab exposure and cancer to ascertain whether the heightened occurrence of malignancy in patients with rheumatoid arthritis currently exposed to rituximab treatment can be exclusively attributed to indication bias.
Conclusion
Disease burden of rheumatoid arthritis remains particularly affected by an increased cancer risk. While smoking related cancers are markedly more incident in treated rheumatoid arthritis patients, particularly in men, women are less at risk of breast, endometrial and pancreatic cancer than the general population. Hematological cancers were still increased in rheumatoid arthritis patients compared to the general population, although incidence tended to decrease with time for non-Hodgkin's lymphoma. Patients exposed to rituximab and abatacept were those who displayed the highest malignancy rate. Knowing these risks, physicians can recommend specific screenings in high risk patients with rheumatoid arthritis which could allow for earlier management in some cases.
Contributors
MB, SP, YD, RS and FT contributed to conceptualization, data curation and formal analysis; MB, BF, XM, RS and FT contributed to data interpretation; MB, RS and FT provided the original draft; MB, BF, XM, RS and FR contributed to final review and editing.
Data sharing statement
Data that support the findings of this study are available upon reasonable request.
Declaration of interests
XM received fees from Astra-Zeneca, BMS, Galapagos, GSK, Novartis, Pfizer; RS received consulting fees from GSK, Bristol Myer Squib, Boerhinger, Amgen, Pfizer and Janssen; travel fees from Amgen and GSK; BF received research grants from AbbVie, Pfizer, Lilly, MSD, and consulting fees from AbbVie, Amgen, Biogen, BMS, Celltrion, Chugai, Fresenius Kabi, Galapagos, Janssen, Lilly, Medac, MSD, NORDIC Pharma, Novartis, OWKIN, Pfizer, Roche, Sandoz, Sanofi-Genzyme, SOBI, UCB, Viatris and support for meetings/travel from Lilly; FT redistributed to her hospital consulting fees from MSD and Novartis.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100768.
Appendix A. Supplementary data
References
- 1.Mercer L.K., Davies R., Galloway J.B., et al. Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatol Oxf Engl. 2013;52(1):91–98. doi: 10.1093/rheumatology/kes350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe F., Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56(5):1433–1439. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 3.Dreyer L., Mellemkjær L., Andersen A.R., et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides - a follow-up study from the DANBIO Registry. Ann Rheum Dis. 2013;72(1):79–82. doi: 10.1136/annrheumdis-2012-201969. [DOI] [PubMed] [Google Scholar]
- 4.Baecklund E., Iliadou A., Askling J., et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 5.Andler R. 2019. Baisse de la prévalence du tabagisme quotidien parmi les adultes: résultats du baromètre de santé publique France 2018/reduction of daily smoking rate among adults: results from the 2018 santé publique France health barometer; p. 7. [Google Scholar]
- 6.Bongartz T., Sutton A.J., Sweeting M.J., Buchan I., Matteson E.L., Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 7.Raaschou P., Simard J.F., Asker Hagelberg C., Askling J., ARTIS Study Group Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ. 2016;352:i262. doi: 10.1136/bmj.i262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montastruc F., Renoux C., Dell'Aniello S., et al. Abatacept initiation in rheumatoid arthritis and the risk of cancer: a population-based comparative cohort study. Rheumatol Oxf Engl. 2019;58(4):683–691. doi: 10.1093/rheumatology/key352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ytterberg S.R., Bhatt D.L., Mikuls T.R., et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 10.Cowppli-Bony A., Colonna M., Ligier K., et al. [Descriptive epidemiology of cancer in metropolitan France: incidence, survival and prevalence] Bull Cancer. 2019;106(7-8):617–634. doi: 10.1016/j.bulcan.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Tuppin P., Rivière S., Rigault A., et al. Prevalence and economic burden of cardiovascular diseases in France in 2013 according to the national health insurance scheme database. Arch Cardiovasc Dis. 2016;109(6):399–411. doi: 10.1016/j.acvd.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Tuppin P., de Roquefeuil L., Weill A., Ricordeau P., Merlière Y. French national health insurance information system and the permanent beneficiaries sample. Rev Épidémiol Santé Publique. 2010;58(4):286–290. doi: 10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Smolen J.S., Landewé R., Breedveld F.C., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajrouche A., Estellat C., De Rycke Y., Tubach F. Evaluation of algorithms to identify incident cancer cases by using French health administrative databases. Pharmacoepidemiol Drug Saf. 2017;26(8):935–944. doi: 10.1002/pds.4225. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F., Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56(9):2886–2895. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama D., Nishimura K., Tamaki K., et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 17.Wadström H., Pettersson A., Smedby K.E., Askling J. Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Ann Rheum Dis. 2020;79(5):581–586. doi: 10.1136/annrheumdis-2019-216756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludvigsson J.F., West J., Ekbom A., Stephansson O. Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer. 2012;131(3):E244–E250. doi: 10.1002/ijc.26454. [DOI] [PubMed] [Google Scholar]
- 19.Salliot C., Nguyen Y., Gusto G., et al. Female hormonal exposures and risk of rheumatoid arthritis in the French E3N-EPIC cohort study. Rheumatol Oxf Engl. 2021;60(10):4790–4800. doi: 10.1093/rheumatology/keab101. [DOI] [PubMed] [Google Scholar]
- 20.Alkhayyat M., Abou Saleh M., Grewal M.K., et al. Pancreatic manifestations in rheumatoid arthritis: a national population-based study. Rheumatology. 2021;60(5):2366–2374. doi: 10.1093/rheumatology/keaa616. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Rubio P., Piñero J., Molina-Montes E., et al. Pancreatic cancer and autoimmune diseases: an association sustained by computational and epidemiological case–control approaches. Int J Cancer. 2019;144(7):1540–1549. doi: 10.1002/ijc.31866. [DOI] [PubMed] [Google Scholar]
- 22.Hellgren K., Di Giuseppe D., Smedby K.E., et al. Lymphoma risks in patients with rheumatoid arthritis treated with biological drugs-a Swedish cohort study of risks by time, drug and lymphoma subtype. Rheumatol Oxf Engl. 2021;60(2):809–819. doi: 10.1093/rheumatology/keaa330. [DOI] [PubMed] [Google Scholar]
- 23.Baecklund E., Smedby K.E., Sutton L.A., Askling J., Rosenquist R. Lymphoma development in patients with autoimmune and inflammatory disorders--what are the driving forces? Semin Cancer Biol. 2014;24:61–70. doi: 10.1016/j.semcancer.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson M. Rheumatoid arthritis as a risk factor for multiple myeloma: a case-control study. Eur J Cancer Oxf Engl. 1993;29A(2):259–263. doi: 10.1016/0959-8049(93)90188-l. [DOI] [PubMed] [Google Scholar]
- 25.Shen K., Xu G., Wu Q., Zhou D., Li J. Risk of multiple myeloma in rheumatoid arthritis: a meta-analysis of case-control and cohort studies. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadström H., Arkema E.V., Sjöwall C., Askling J., Simard J.F. Cervical neoplasia in systemic lupus erythematosus: a nationwide study. Rheumatol Oxf Engl. 2017;56(4):613–619. doi: 10.1093/rheumatology/kew459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long M.D., Martin C.F., Pipkin C.A., Herfarth H.H., Sandler R.S., Kappelman M.D. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390–399.e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huss V., Bower H., Wadström H., et al. Short- and longer-term cancer risks with biologic and targeted synthetic disease-modifying antirheumatic drugs as used against rheumatoid arthritis in clinical practice. Rheumatology. 2022;61(5):1810–1818. doi: 10.1093/rheumatology/keab570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedra J., Seror R., Dieudé P., et al. Lymphoma complicating rheumatoid arthritis: results from a French case-control study. RMD Open. 2021;7(3) doi: 10.1136/rmdopen-2021-001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs J.D., Cohen S.B., Emery P., et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis. 2013;72(3):329–336. doi: 10.1136/annrheumdis-2011-201117. [DOI] [PubMed] [Google Scholar]
- 31.Griss J., Bauer W., Wagner C., et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. 2019;10(1):4186. doi: 10.1038/s41467-019-12160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadström H., Frisell T., Askling J. Anti-rheumatic therapy in Sweden (ARTIS) study group. Malignant Neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605–1612. doi: 10.1001/jamainternmed.2017.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozen G., Pedro S., Schumacher R., Simon T.A., Michaud K. Safety of abatacept compared with other biologic and conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: data from an observational study. Arthritis Res Ther. 2019;21(1):141. doi: 10.1186/s13075-019-1921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pina Vegas L., Drouin J., Dray-Spira R., Weill A. Prevalence, mortality, and treatment of patients with rheumatoid arthritis: a cohort study of the French National Health Data System, 2010-2019. Joint Bone Spine. 2023;90(1) doi: 10.1016/j.jbspin.2022.105460. [DOI] [PubMed] [Google Scholar]
- 35.Guillemin F., Saraux A., Guggenbuhl P., et al. Prevalence of rheumatoid arthritis in France: 2001. Ann Rheum Dis. 2005;64(10):1427–1430. doi: 10.1136/ard.2004.029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.