Introduction
Globally, over 13 million individuals undergo digestive surgery each year1. Digestive surgery remains associated with a substantial risk of postoperative complications2, which has a detrimental impact on costs and on caregivers3. Efforts to accurately predict postoperative complications could reduce their impact, and considerable attempts have been made to hone this predictive ability. Unfortunately, results have shown limited performance4.
Artificial intelligence (AI) is the broader concept of machines being able to execute tasks intelligently, while machine learning (ML) is a distinct branch of AI that involves training machines to optimize their performance through exposure to data, using algorithms, such as artificial neural networks5. Its potent contributions have substantially impacted various fields, including healthcare6–10. The aim of the present scoping review was to provide an overview of the available data investigating ML to predict postoperative complications after digestive surgery.
Methods
This prospectively registered review was conducted in accordance with the current authoritative frameworks for scoping reviews, including studies on the use of ML to predict postoperative complications in digestive surgery. A detailed description of the methods is provided in the Supplementary Methods.
Results
Study selection
A search of the literature yielded 4327 records. After the application of inclusion and exclusion criteria, a total of 53 articles met the eligibility criteria (Fig. S1). Table 1 summarizes these 53 studies.
Table 1.
Overview of selected study characteristics
| Number of studies | Sample size, median (interquartile range) | Common POC | ML versus CS | |
|---|---|---|---|---|
| Upper-GI |
|
4334 (919–44 061) |
|
ML > CS in 3 of 4 studies |
| HPB |
|
552 (159–1322) |
|
ML > CS in 5 of 7 studies |
| Colorectal |
|
944 (244–3956) |
|
ML > CS in 8 of 8 studies |
| General digestive |
|
2372 (926–68 224) |
|
ML > CS in 4 of 6 studies |
| Total |
|
1137 (269–5824) |
|
ML > CS in 20 of 25 studies |
POC; postoperative complications; ML, machine learning; CS, conventional statistics; GI, gastrointestinal; AL, anastomotic leakage; HPB, hepatopancreatobiliary; POPF, postoperative pancreatic fistula; PHLF, post-hepatectomy liver failure, AKI, acute kidney injury; SSI, surgical site infection; DS, digestive surgery.
Characteristics of sources of evidence
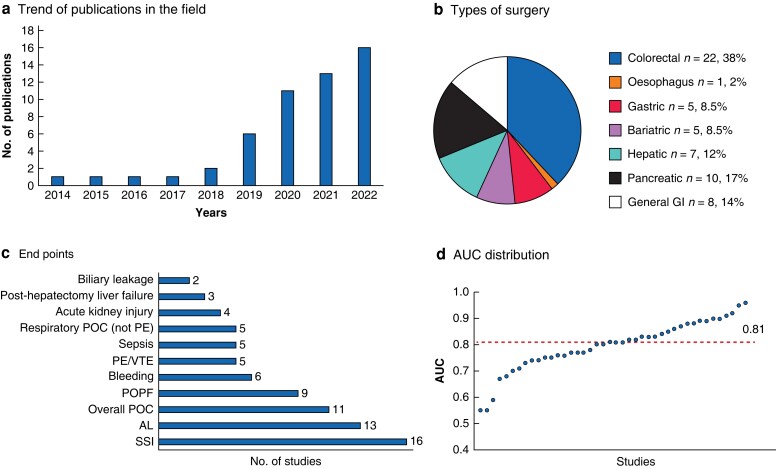
The topic has gained major interest over the last years, with most studies (47 of 53, 87 per cent) being published from 2019 onwards (Fig. 1a). The distribution of these studies based on the type of surgery is detailed in Fig. 1b. The most frequently investigated endpoints are illustrated in Fig. 1c. Sample sizes were heterogeneous, ranging from 3211 to 1 003 703 patients12. Various ML algorithms were established, including artificial neural networks (24, 45 per cent), gradient-boosted machines (24, 45 per cent), and random forests (22, 42 per cent). The area under the curve (AUC) of the model was provided by 44 studies (83 per cent), showing a median value of 0.81 (0.75–0.87) (Fig. 1d), and compared with conventional statistical methods in 25 (47 per cent) studies.
Fig. 1.
Characteristics of the selected studies
a Number of articles published per year on the topic. b Distribution of the selected studies according to the types of digestive surgery. c Selected endpoints and their frequency. d Distribution of the values of the area under the curve for the reported machine-learning models that aimed to predict postoperative complications after digestive surgery. GI, gastrointestinal; POC, postoperative complications; PE, pulmonary embolism; VTE, venous thromboembolism; POPF, postoperative pancreatic fistula; AL, anastomotic leakage; SSI, surgical site infection; AUC, area under the curve.
Upper-gastrointestinal surgery
A total of 10 articles were identified, with 5 studies involving bariatric surgery, 4 studies involving gastric surgery and 1 study involving oesophagogastric surgery (Table S2). Anastomotic leakage (AL) is an important issue after upper-gastrointestinal surgery, associated with substantial consequences. Integrating demographics, medical history, laboratory tests, and surgical details, Shao et al.13 established an ML model to predict AL after gastrectomy, showing a good performance, with an AUC of 0.90. In a large cohort of patients undergoing bariatric surgery, ML showed a better predictive value for AL compared with a linear model (AUC 0.75 versus 0.63 respectively, P < 0.001)14. Nudel et al.14 utilized a nationwide database from the USA to analyse the predictive value of ML for AL and venous thromboembolism (VTE) after bariatric surgery. For both types of complications, ML outperformed linear models (AUC = 0.75 versus 0.63 respectively for AL and AUC = 0.67 versus 0.64 respectively for VTE).
Hepatopancreatobiliary surgery
A total of 13 studies were included in this section (Table S3). Preoperative imaging is often available for patients undergoing pancreatic surgery and this type of data can be used to predict surgical outcomes. Among the studies that applied imaging to postoperative pancreatic fistula (POPF) prediction algorithms, three groups of researchers integrated imaging data in ML algorithms and showed promising predictive values. As an example, a proof-of-concept of this approach was investigated in a pilot study of 110 patients undergoing pancreatoduodenectomy, equally matched for POPF (55 patients with POPF and 55 patients without POPF)15. The imaging-based model showed excellent performance, with an AUC of 0.95, with a sensitivity and specificity of 96 and 98 per cent respectively.
A total of five studies (38 per cent) were conducted in patients undergoing liver surgery; three of these studies (60 per cent) explored the application of ML models to tackle the challenging complication of post-hepatectomy liver failure (PHLF). In a cohort of 353 patients with hepatocellular carcinoma (‘HCC’), ML showed a valuable performance, with an AUC of 0.88 compared with 0.79 (P < 0.050) in a linear model16.
Colorectal surgery
A total of 20 studies were included in this section (Table S4). The Mayo Clinic group used the American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) to build an ML model that aimed to predict surgical site infection after colorectal surgery17. They reported a good performance, with an AUC of 0.83, which outperformed a linear model (AUC = 0.72). Other studies leveraged ML to predict AL, showing promising results. As an illustration, an algorithm developed in a cohort of 5220 patients undergoing anterior resection for rectal cancer showed an AUC of 0.87 to predict postoperative AL, as opposed to an AUC of 0.72 for linear regression18.
General digestive surgery
This section included 10 studies (Table S5). A total of four of six (66 per cent) studies comparing ML with linear models highlighted the higher performance of the ML algorithms.
In a large-scale study analysing 246 124 patients from the NSQIP database (197 488 patients for colectomy, 25 403 for hepatectomy, and 23 333 for PD), AL advantageously predicted biliary leakage (AUC = 0.75 versus 0.72, P < 0.001), POPF (AUC = 0.75 versus 0.71, P = 0.003), and AL (AUC = 0.68 versus 0.63, P = 0.001) compared with linear regression19.
The ML-based Predictive OpTimal Trees in Emergency surgery Risk (‘POTTER’) calculator is an externally validated risk-assessment tool, which was also developed from the ACS-NSQIP database, which showed promising performance in predicting mortality and morbidity20.
Discussion
The use of ML to predict postoperative complications after digestive surgery found 53 studies that demonstrated a feasible and promising approach. Moreover, ML appeared as a polyvalent tool capable of predicting different types of postoperative complications in various settings.
Despite growing interest and a rise in publications over the past 5 years, data on the subject remain scant. Furthermore, the included studies showed significant heterogeneity. While ML may offer superior performance, its success hinges on the quality of input data. Thus identifying new potent biomarkers is paramount for improving the prediction of postoperative complications, a challenge that ML alone cannot solve. Also, ML offers unique opportunities to exploit new sources of input data for the prediction of postoperative complications, such as intraoperative video samples. The heterogeneous designs translated into heterogeneous performances of the models, with a wide range of AUC values. Nonetheless, the median AUC reached the encouraging value of 0.81, and ML showed a higher performance than linear models in the majority of available comparisons (20 of 25).
Future efforts in the field must focus on conducting studies including independent cohort for external validation. Particularly, the clinical impact and standard requirements for performance, such as AUC values, must be investigated and determined. Also, examining ML performance across subgroups, namely oncological/non-oncological and elective/emergent patients, could expose the need for distinct algorithms adapted to specific clinical scenarios. Many included studies lacked rigorous and transparent descriptions of ML algorithm development and data preparation methods. This is critical because the same algorithms can produce different outcomes based on their implementation, emphasizing the need for in-depth understanding to progress in future research.
Supplementary Material
Acknowledgements
Author contributions: study concept and design, M.R. and I.L.; acquisition of data, M.R., G.-R.J., and I.L; analysis and interpretation of data, M.R., G.-R.J., and I.L.; drafting of the manuscript, M.R. and I.L.; and critical revision of the manuscript for important intellectual content, M.R., G.-R.J., N.D., E.U., E.M., and I.L.
Contributor Information
Maximilien Ravenel, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.
Gaëtan-Romain Joliat, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland; Graduate School of Health Sciences, University of Bern, Bern, Switzerland.
Nicolas Demartines, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.
Emilie Uldry, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.
Emmanuel Melloul, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.
Ismail Labgaa, Department of Visceral Surgery, Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Lausanne, Switzerland; Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL), Lausanne, Switzerland.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data used in this article are already publicly available.
References
- 1. Rose J, Weiser TG, Hider P, Wilson L, Gruen RL, Bickler SW. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health 2015; 3(Suppl 2): S13–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser Det al. . The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–913 [DOI] [PubMed] [Google Scholar]
- 3. Pinto A, Faiz O, Bicknell C, Vincent C. Surgical complications and their implications for surgeons’ well-being. Br J Surg 2013;100:1748–1755 [DOI] [PubMed] [Google Scholar]
- 4. Moonesinghe SR, Mythen MG, Das P, Rowan KM, Grocott MPW. Risk stratification tools for predicting morbidity and mortality in adult patients undergoing major surgery. Anesthesiology 2013;119:959–981 [DOI] [PubMed] [Google Scholar]
- 5. Bishop CM, Nasrabadi NM. Pattern Recognition and Machine Learning. Vol. 4. New York: Springer, 2006, 1–4 [Google Scholar]
- 6. Rahman SA, Walker RC, Lloyd MA, Grace BL, van Boxel GI, Kingma BFet al. . Machine learning to predict early recurrence after oesophageal cancer surgery. Br J Surg 2020;107:1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohr A, Memarzadeh K. The rise of artificial intelligence in healthcare applications. Artificial Intelligence in Healthcare 2020:25–60. doi: 10.1016/B978-0-12-818438-7.00002-2 [DOI] [Google Scholar]
- 8. Gögenur I. Introducing machine learning-based prediction models in the perioperative setting. Br J Surg 2023;110:533–535 [DOI] [PubMed] [Google Scholar]
- 9. Soh CL, Shah V, Arjomandi Rad A, Vardanyan R, Zubarevich A, Torabi Set al. . Present and future of machine learning in breast surgery: systematic review. Br J Surg 2022;109:1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. COVIDSurg Collaborative . Machine learning risk prediction of mortality for patients undergoing surgery with perioperative SARS-CoV-2: the COVIDSurg mortality score. Br J Surg 2021;108:1274–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sofo L, Caprino P, Schena CA, Sacchetti F, Potenza AE, Ciociola A. New perspectives in the prediction of postoperative complications for high-risk ulcerative colitis patients: machine learning preliminary approach. Eur Rev Med Pharmacol Sci 2020;24:12781–12787 [DOI] [PubMed] [Google Scholar]
- 12. Hadaya J, Verma A, Sanaiha Y, Ramezani R, Qadir N, Benharash P. Machine learning-based modeling of acute respiratory failure following emergency general surgery operations. PLoS One 2022;17:e0267733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shao S, Liu L, Zhao Y, Mu L, Lu Q, Qin J. Application of machine learning for predicting anastomotic leakage in patients with gastric adenocarcinoma who received total or proximal gastrectomy. J Pers Med 2021;11:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nudel J, Bishara AM, de Geus SWL, Patil P, Srinivasan J, Hess DTet al. . Development and validation of machine learning models to predict gastrointestinal leak and venous thromboembolism after weight loss surgery: an analysis of the MBSAQIP database. Surg Endosc 2021;35:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kambakamba P, Mannil M, Herrera PE, Müller PC, Kuemmerli C, Linecker Met al. . The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: a proof-of-principle study. Surgery 2020;167:448–454 [DOI] [PubMed] [Google Scholar]
- 16. Mai RY, Lu HZ, Bai T, Liang R, Lin Y, Ma Let al. . Artificial neural network model for preoperative prediction of severe liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Surgery 2020;168:643–652 [DOI] [PubMed] [Google Scholar]
- 17. Sohn S, Larson DW, Habermann EB, Naessens JM, Alabbad JY, Liu H. Detection of clinically important colorectal surgical site infection using Bayesian network. J Surg Res 2017;209:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen R, Zheng K, Zhang Q, Zhou L, Liu Q, Yu Get al. . Machine learning-based random forest predicts anastomotic leakage after anterior resection for rectal cancer. J Gastrointest Oncol 2021;12:921–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen KA, Berginski ME, Desai CS, Guillem JG, Stem J, Gomez SMet al. . Differential performance of machine learning models in prediction of procedure-specific outcomes. J Gastrointest Surg 2022;26:1732–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA. Surgical risk is not linear: derivation and validation of a novel, user-friendly, and machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) calculator. Ann Surg 2018;268:574–583 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this article are already publicly available.