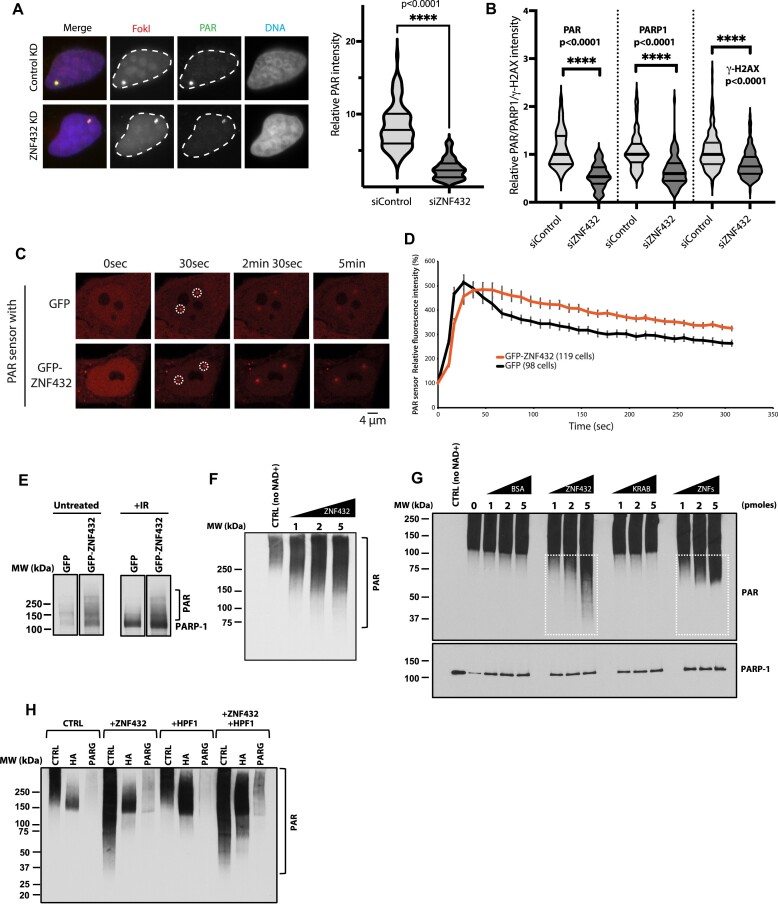
Figure 5.
ZNF432 promotes PARP-1 assembly and activation. (A) Left: Knockdown of ZNF432 reduces PARylation at localized DSBs. i265 U2OS cells were transfected with control or ZNF432 siRNAs for 7 days before being analyzed at 1 h after DSB induction. The PAR signal is shown in green, while the DSB sites were marked by mCherry-FokI in red. Nuclei were stained by Hoechst 33342 in blue. Right: A violin plot displaying the quantification of relative PAR intensity in siControl or siZNF432 cells in the experimental setting of Figure 5A. The dotted line indicates the mean. The slide line below the mean labels the 1st quartile and the slide line above the mean demarcate the 3rd quartile, respectively. P-values were calculated by unpaired two-tailed Student's t-test as shown. (B) i265 U2OS cells transfected with siRNAs (control siRNA and ZNF432 smart pool) were processed at 1 h post-DSB induction for immunofluorescence. The violin plot represents the quantification of the relative intensity of PAR, PARP-1 and γ-H2AX foci on the array. (C) Overexpression of GFP-ZNF432 increase PARylation as measured with a PBZ-mRuby PAR sensor. (D) Monitoring of the mRuby-PAR relative fluorescence intensity over time. (E) ZNF432 stimulates PARP-1 activity. Overexpression of GFP-ZNF432 increases intracellular PAR levels under basal conditions and in IR-treated cells. Whole cell extracts of ZNF432-expressing cells were analyzed for the presence of PAR by western blot. (F) Western blot showing the in vitro automodification pattern of PARP-1 in the presence of full length human ZNF432 (1, 2 and 5 pmoles). PARP-1 was incubated with ZNF432, calf-thymus activated DNA and NAD+. Reaction products were resolved by SDS-PAGE and PAR polymers were identified by western blot. A polyclonal antibody against PAR (96–10) was used in both blots E and F to reveal the presence of PAR polymers. (G) The ZNFs region of ZNF432 stimulates PARP-1 activity. The Western blot shows the in vitro auto modification pattern of PARP-1 in the presence of bovine serum albumin (BSA, control), full length ZNF432 (1–652), the N-terminal fragment containing the KRAB domain (1–205) and the C-terminal domain containing the array of 16 ZNFs repeats (205–652). PAR polymers were revealed by Western blot analysis using a polyclonal antibody against MAR/PAR (E6F6A). (H) ZNF432 and HPF1 produce an additive stimulatory effect on PARP-1 activity. PARP-1 was auto modified in vitro in the presence of ZNF432, HPF1 and both proteins. Reaction products were subjected to 1M hydroxylamine (HA) hydrolysis to cleave Asp/Glu-linked ADP-ribosylation or PARG treatment to erase PAR polymers. PAR polymers were revealed by Western blot analysis using a polyclonal antibody against PAR (96–10).