Abstract
Carotenoids are produced by a variety of organisms, but the mechanisms that regulate gene expression leading to carotenoid biosynthesis have been characterized for only a few organisms. In this study, we found that Streptomyces coelicolor A3(2), a gram-positive filamentous bacterium, produces carotenoids under blue light induction. The carotenoid fraction isolated from the cell extract contained multiple compounds, including isorenieratene and β-carotene. The carotenoid biosynthesis gene cluster of S. coelicolor consists of two convergent operons, crtEIBV and crtYTU, as previously shown for Streptomyces griseus. The crtEIBV null mutant completely lost its ability to produce carotenoids. The crt gene cluster is flanked by a regulatory region that consists of two divergent operons, litRQ and litSAB. The lit (light-induced transcription) genes encode a MerR-type transcriptional regulator (LitR), a possible oxidoreductase (LitQ), an extracytoplasmic function sigma factor (σLitS), a putative lipoprotein (LitA), and a putative anti-sigma factor (LitB). S1 protection assay revealed that the promoters preceding crtE (PcrtE), crtY (PcrtY), litR (PlitR), and litS (PlitS) are activated upon illumination. A litS mutant lost both the ability to produce carotenoids and the activities of PcrtE, PcrtY, and PlitS, which suggested that σLitS directs light-induced transcription from these promoters. An RNA polymerase holocomplex containing purified σLitS recombinant protein generated specific PcrtE and PcrtY transcripts in an in vitro runoff transcriptional assay. A litR mutant that had an insertion of the kanamycin resistance gene was defective both in the ability to produce carotenoids and in all of the light-dependent promoter activities. Overexpression of litS resulted in constitutive carotenoid production in both the wild type and the litR mutant. These results indicate that σLitS acts as a light-induced sigma factor that directs transcription of the crt biosynthesis gene cluster, whose activity is controlled by an unknown LitR function. This is the first report to describe light-inducible gene expression in Streptomyces.
Carotenoids are pigmented compounds that are widely produced by both eucaryotes and procaryotes (11). They are tetraterpenoids that consist of a polyene hydrocarbon chain derived from eight isoprene units. The C40 backbone is modified in several ways, such as cyclization and desaturation, to produce a variety of compounds with divergent chemical structures. Carotenoids have two major roles within the cell. First, in photoautotrophic organisms, they act as accessory pigments in the light-capturing complexes by absorbing light in the wavelength range of 400 to 500 nm and transferring the energy to chlorophyll. Second, in both phototrophic and nonphotoautotrophic organisms, they protect cells from photo-oxidative damage by scavenging harmful agents such as singlet and triplet molecular species produced upon illumination.
Carotenogenesis in prokaryotes is constitutive or photoinducible. Several prokaryotes, including Erwinia herbicola (1) and Rhodobacter capsulatus (3) produce carotenoids constitutively whereas organisms such as Myxococcus xanthus (9), Flavobacterium dehydrogenans (28), and Sulfolobus spp. (12) produce carotenoids in a photoinducible manner. The control mechanisms of carotenogenesis have been studied in phototrophic bacteria such as Rhodobacter spp., which has revealed the involvement of global signal transduction initiated by light capture in the bacteriochlorophyll (2). On the other hand, the molecular mechanism in nonphototrophic bacteria has not yet been fully studied except in M. xanthus, a gram-negative gliding bacterium characterized by a unique life cycle. In M. xanthus, extensive studies have revealed a major signaling pathway from light sensing to transcriptional control of biosynthetic genes, which involves a light-dependent extracytoplasmic function (ECF) sigma factor, CarQ, and its cognate membrane-associated anti-sigma factor, CarR (6, 8, 10, 23, 29).
We study carotenogenesis in Streptomyces, a gram-positive bacterial genus renowned for its ability to produce a variety of secondary metabolites. Although it is well known that the carotenoid-producing ability is widespread in this group of bacteria, the molecular mechanism involved in the regulation of carotenoid production is poorly characterized. We have found that carotenogenesis is light inducible in Streptomyces coelicolor A3(2), the model organism for genetic analysis. While the information from genomic sequence data (5) has already shown that this organism retains a carotenoid biosynthesis gene cluster, until now this has not been demonstrated to lead to carotenoid production. In normal strains of S. coelicolor A3(2), it is not clear whether the organisms produce carotenoids, since the marked production of actinorhodin and prodigiosin, the blue diffusible and red intracellular pigment antibiotics, respectively, prevents clear observation of the yellow pigment. Meanwhile, we noticed that an S. coelicolor strain harboring pKM284, a plasmid that has an activity which represses the production of the two pigmented antibiotics, accumulated the yellow pigment in the cell when it was illuminated with visible light. We also found that the photodependent carotenoid production occurs in the parental S. coelicolor strain. Here, we report the light-induced transcription of the carotenoid biosynthesis gene cluster of this organism along with the identification of a responsible sigma factor and related regulators.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
S. coelicolor A3(2) M145 (wild type), obtained from the John Innes Institute (Norwich, United Kingdom), was used as a parental strain and designated the wild type. The isolation of pKM284, the plasmid that repressed antibiotic production in wild-type S. coelicolor, was described previously (27). Escherichia coli JM109 (24) and BL21(DE3) (Novagen) were used as hosts for DNA manipulation and expression of recombinant proteins. pUC19 (30) was used for general DNA manipulation. pT7Blue (Novagen) was used for TA cloning of PCR products. pGEX4T-2 (Pharmacia) and pET26b(+) (Novagen) were used as vectors for effective expression of recombinant proteins. The conditions used for genetic manipulation in E. coli and Streptomyces have been described by Maniatis et al. (24) and Kieser et al. (18), respectively. All vector plasmids used were described by Kieser et al. (18). Cosmid StJ12, which contains the crt locus, was obtained from H. Kieser (John Innes Institute). S. coelicolor A3(2) strains were grown in Bennett's sugar medium (containing [in grams per liter] the following: yeast extract [Difco Laboratories, Detroit, Mich.], 1; meat extract [Kyokuto, Tokyo, Japan], 1; NZ amine [Wako Pure Chemical Industries, Ltd., Tokyo, Japan], 2; and an appropriate sugar [Kokusan, Tokyo, Japan], 10 [pH 7.2]) and YMP-sugar medium (containing [in grams per liter] the following: yeast extract [Difco], 2; meat extract [Kyokuto], 2; Bacto Peptone [Difco], 4; NaCl, 5; MgSO4 · 7H2O, 2; and an appropriate sugar [Kokusan], 10 [pH 7.2]). Solid media contained 1.5% agar (Kokusan). In order to select E. coli transformants, ampicillin (Wako) and kanamycin (Wako) were used at a final concentration of 50 μg/ml, and for selecting S. coelicolor A3(2) transformants, thiostrepton (Sigma Chemical Co., St. Louis, Mo.) and kanamycin were added to a final concentration of 20 μg/ml. Enzymes used for genetic manipulation were purchased from Takara-shuzo (Kyoto, Japan).
Production, extraction, and identification of carotenoids.
In order to collect cells, S. coelicolor A3(2) strains were grown on cellophane on the surface of Bennett's glucose solid medium for 5 days at 28°C under light or dark conditions in a darkroom equipped with white light fluorescent lamps (Toshiba) (15 W). Under light conditions, white light was illuminated at approximately 2.4 μmol s−1 m−2 onto the solid culture. The same lamp, covered with a blue or red light filter, was used for illumination of blue (maximum transmittance of between 400 and 460 nm) or red (600 to 700 nm) light, respectively.
The carotenoid fraction was extracted by the method described by Schumann et al. (25). Cells collected from five agar plates were first lyophilized and then suspended in 10 ml of chloroform and incubated at 60°C for 15 min with occasional vortex mixing. The sample was then placed on ice for 5 min, filtered to remove cell debris, and evaporated. After drying, the sample was redissolved in 10 μl of chloroform. The concentrated preparation was subsequently purified on a silica gel column (Bond Elut; Varian) and eluted with hexane-toluene (85:15), according to the manufacturer's protocol, in order to obtain the final carotenoid fraction.
The resulting carotenoid fraction was subjected to photometric analysis with a UV spectrometer (UVmini-1240; Shimadzu). Qualitative analyses were performed by silica gel thin-layer chromatography with a solvent mixture of methanol-acetonitrile-2-propanol (85:10:5) for development and by silica gel column chromatography with high-performance liquid chromatography (HPLC) (LC-10; Shimadzu), as described by Schumann et al. (25). An authentic β-carotene sample was purchased from Funakoshi (Tokyo, Japan) and used as the standard control. Isorenieratene was isolated from cell extracts of Mycobacterium phlei JCM5865 as described previously (21).
S1 nuclease mapping.
Transcriptional activities of the promoters preceding crtE (PcrtE), crtY (PcrtY), litQ (PlitQ), litR (PlitR), and litS (PlitS) were examined by S1 protection assays. Preparation of RNA from cells grown on cellophane on the surface of agar medium and S1 nuclease mapping were performed as described by Kelemen et al. (17). Hybridization probes were prepared by PCR with various oligonucleotide primers (Table 1): PEF and PER (PcrtE, low resolution), PEF and PERH (PcrtE, high resolution), PYF and PYR (PcrtY, low resolution), PYF and PYRH (PcrtY, high resolution), PQF and PQR (PlitQ, low resolution), PQF and PQRH (PlitQ, high resolution), PRF and PRR (PlitR, low resolution), PRF and PRRH (PlitR, high resolution), PSF and PFR (PlitS, low resolution), PFF and PFRH (PlitS, high resolution), PAF and PAR (litA, readthrough), and PBF and PBR (litB, readthrough). Primers PRF and aph were used to generate the hybridization probe for investigating PlitR activity in the litR mutant that carries an aphII gene cassette downstream of the promoter region. In all probes, the downstream primers were labeled at the 5′ ends with [γ-32P]ATP by using T4 polynucleotide kinase. S1-protected fragments were analyzed on 6% polyacrylamide gels. Maxam-Gilbert sequence ladders prepared from the labeled hybridization probe were used as standards for high-resolution analysis. Protected fragments were analyzed on a 6% polyacrylamide gel. The quality of RNA used for low-resolution analysis was checked by a control assay for hrdB, encoding a major sigma factor, using a probe described previously (19).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′)a | Restriction enzyme | Position (nucleotides)b |
|---|---|---|---|
| PEF | 5′-AGCTGCTGGCCGGCAATCAGC | 173554-173574 | |
| PER | 5′-TCGACCGCGTTCGCCGCATCG | 173886-173866 | |
| PERH | 5′-ACCATCGGCGGCAGTCCAGG | 173823-173804 | |
| PYF | 5′-TACGTGTGCGGAGACCACCGG | 182413-182393 | |
| PYR | 5′-ACACTCTTCGGTTCCACGCTTC | 182194-182215 | |
| PYRH | 5′-TGCCACCGGGTCACGCACAC | 182178-182197 | |
| PQF | 5′-TGACCCTGGGCCCTGGATGG | 183896-183877 | |
| PQR | 5′-TGATGCCCAGGGGCAAAGG | 183587-183605 | |
| PQRH | 5′-TGCGAGTGCGGGGAAACG | 183675-183692 | |
| PRF | 5′-TCGTGTACGCGTGTTCATGC | 184975-184956 | |
| PRR | 5′-ACCTCGCCGGTGGTCAATCC | 184718-184737 | |
| PRRH | 5′-TCGTCGACCGGCTCGTCG | 184757-184774 | |
| PSF | 5′-ACTATACGACGCACAAACGACG | 184804-184825 | |
| PSR | 5′-TTCCTCCTGCCGTGCGTCGTACG | 184952-184930 | |
| PSRH | 5′-ACGTCGCCGGTCTACCCTGC | 184901-184882 | |
| PAF | 5′-TTCTACGAGGACCTGACGCAGG | 185426-185447 | |
| PAR | 5′-TTGGCGAAGACGTCGACCGTCG | 185835-185814 | |
| PBF | 5′-ACCAGACTCTGGCTCTGCCCG | 186213-186233 | |
| PBR | 5′-TCGCCACTGGCGTGACCGAGTGC | 186541-186519 | |
| PhrdF | 5′-CGGCCGCAAGGTACGAGTTGATGA | 78385-78408 | |
| PhrdR | 5′-GCCATGACAGAGACGGACTCGGCG | 78726-78703 | |
| aph | 5′-TGATATTGCTGAAGAGCTTGG | 2228-2248 | |
| rSF | 5′-GGAACGCATATGAACACCCGTACCCGCCCGAC | NdeI | 184949-184980 |
| rSR | 5′-GGCCAAGCTTGACACACGTGTC | HindIII | 185573-185552 |
| DcrtF | 5′-CTGGAAGCTTTACTGGTACT | HindIII | 173081-173099 |
| DcrtMR | 5′-ATCAGATCTTCGTGGACCAGAGC | Bg/II | 174132-174110 |
| DcrtMF | 5′-TTTAGATCTCGACGTCTCCTTCC | Bg/II | 178146-178168 |
| DcrtR | 5′-CGCGGAATTCTGCTGATGTTCC | EcoRI | 179422-179400 |
| DRF | 5′-CGTCGAATTCCTGCCGCCGAAG | EcoRI | 186262-186241 |
| DRMR | 5′-TACGAGATCTATGCCCACTATAC | Bg/II | 184787-184810 |
| DRMF | 5′-CCCGAGATCTGTTGTCGTCC | Bg/II | 183799-183780 |
| DRR | 5′-GCACAAGCTTCAGGATGGCTCCG | HindIII | 182651-182672 |
| DSF | 5′-CCCGTCCCCGTACGACGCACGGCAGGAGGAACGGGCATGATTCCGGGGATCCGTCGACC | 184922-184960 | |
| DSR | 5′-GTGGATGCGCCCCGGGGCGTCTCTGCTCCGGCCCGGTCATGTAGGCTGGAGCTGCTTC | 185602-185564 | |
| DBF | 5′-CCGGGAATTCACGAGGTACTC | EcoRI | 185336-185356 |
| DBMR | 5′-GTGAAGATCTGCGCCCTCCTG | Bg/II | 186491-186471 |
| DBMF | 5′-GAACAGATCTCCGAGGTCCG | Bg/II | 186881-186900 |
| DBR | 5′-GAGGAAGCTTAGTACGTCCTCC | HindIII | 188594-188573 |
| DSABF | 5′-CATAAAGCTTCTCACTCGACG | HindIII | 183557-183577 |
| DSABMR | 5′-TACGAGATCTTCAGCCCGTTCC | Bg/II | 184970-184952 |
| HRF | 5′-GTGGGCATGCACGCGTACACG | SphI | 184805-184783 |
| HRR | 5′-GACAAGATCTCACGGGTACACC | Bg/II | 183784-183805 |
| HSF | 5′-ACGGGCATGCACACGCGTAC | SphI | 184952-184971 |
| HSR | 5′-TGGGAGATCTCGAAGGTGTTTCG | Bg/II | 185640-185618 |
Restriction sites are underlined.
All position numbers except those for primer aph correspond to the numbering in the S. coelicolor genomic sequence (http://www.sanger.ac.uk/Projects/S_coelicolor). For aph, the numbering corresponds to the description given by Beck et al.(4).
Gene disruption and overexpression.
The kanamycin-resistant crtEIBV, litR, litAB, and litB mutants were generated by standard homologous recombination techniques that exchanged the wild-type allele with a mutated construct on a disruption plasmid. In order to generate the crtEIBV null mutant, the flanking DNA fragments were PCR amplified with the primers DcrtF-DcrtMR and DcrtMF-DcrtR (Table 1), and these fragments were digested with HindIII-BglII and BglII-EcoRI, respectively, and inserted between the EcoRI and HindIII sites of pUC19 by three-fragment ligation. The resulting plasmid was cleaved with BglII and ligated to a 0.9-kb aphII (kanamycin resistance) (4) cassette to generate the disruption plasmid. This construction replaced the entire crtEIBV region with aphII, which is transcribed in the opposite direction to crtEIBV. Similarly, each of the two DNA fragments prepared with primers DRF-DRMR and DRMF-DRR (for disruption of litR), DSABF-DSABMR and DBMF-DBR (for disruption of litAB), and DBF-DBMR and DBMF-DBR (for disruption of litB) was processed to generate kanamycin-resistant disruption plasmids. The disruption plasmids were introduced into S. coelicolor A3(2) wild-type cells by standard transformation; subsequently, we screened for kanamycin-resistant colonies. True recombination was confirmed by Southern hybridization. The resultant kanamycin-resistant disruptants carried an aphII cassette in each lit coding sequence in the opposite orientation (litR) and the same orientation (litAB and litB) as the disrupted lit gene. We employed REDIRECT technology for markerless disruption of litS; primers DSF and DSR were used to amplify the litS coding sequence from the cosmid StJ12, which was then processed as described by Gust et al. (13) to generate a markerless litS mutant. The resultant litS mutant lacked the entire litS coding sequence.
In order to overexpress LitS and LitR, the DNA fragments were amplified with primers HSF-HSR and HRF-HRR, respectively, and these fragments were digested with SphI and BglII and ligated between the SphI and BglII sites of pIJ702 (16). The resulting plasmids carried the promoterless coding sequences of litR and litS downstream of the mel promoter and in the same orientation, such that the promoter directs constitutive transcription of these genes.
Expression and purification of LitS by an E. coli host-vector system.
The coding sequence of litS was amplified with primers rSF and rSR (Table 1) and cloned between the NdeI and HindIII sites of pET26b. This plasmid construct directed the expression of σLitS as a fusion protein with a C-terminal histidine tag in E. coli BL21(DE3). The E. coli cells harboring the expression plasmid were aerobically cultured at 28°C in 100 ml of Luria-Bertani liquid medium (24), and when the optical density at 600 nm reached 0.8, IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM) was added. Following IPTG addition, the cells were further grown for 4 h and then harvested by centrifugation. The resulting cell pellet was resuspended in an appropriate volume of phosphate-buffered saline buffer (24) and disrupted by sonication. The soluble recombinant proteins were purified from the cell extract by Ni affinity chromatography according to the manufacturer's instructions.
In vitro runoff transcription.
An in vitro runoff transcription assay was performed by a previously described method (26). DNA fragments that contained the PcrtE, PcrtY, and PlitS regions were used as templates. These fragments were PCR amplified with the primers PEF and PER (PcrtE), PYF and PYR (PcrtY), and PSF and PSR (PlitS). These PCR products were mixed with the E. coli RNA polymerase core enzyme (AR Brown) and purified σLitS protein, which was prepared as described above. The transcripts that were generated were analyzed by polyacrylamide gel electrophoresis. A 100-bp ladder marker (Takara-shuzo), denatured by heat treatment, was used as a standard to estimate transcript sizes.
RESULTS
Characterization of photodependent carotenogenesis in S. coelicolor A3(2).
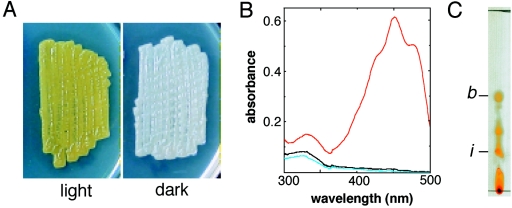
During our study on the effect of pKM284, which was previously isolated by its repression of aerial growth of Streptomyces griseus (27), we noticed that the introduction of this plasmid into S. coelicolor A3(2) abolished the production of actinorhodin and prodigiosin, the pigmented antibiotics usually produced by this organism. The pigmented-antibiotic-deficient transformant in turn clearly exhibited the accumulation of yellow pigment only when it was illuminated (Fig. 1A). The yellow color and light induction suggested that the pigment was a carotenoid(s).
FIG. 1.
Carotenoid production by S. coelicolor A3(2). (A) Colonies of wild-type S. coelicolor harboring pKM284. Colonies grown for 4 days on Bennett's glucose solid medium under light and dark conditions are shown. This strain is defective in the production of blue- and red-pigmented antibiotics due to the unknown effect of pKM284; consequently, it clearly exhibits the accumulation of yellow carotenoid pigments in the cells. (B) UV-visible spectrum of the purified carotenoid fraction extracted from S. coelicolor wild-type cells grown for 2 days under light (red line) and dark (black line) conditions. The same fraction extracted from the crtEIBV mutant cultured under light conditions was also analyzed (blue line). (C) Thin-layer chromatography analysis of the purified carotenoid fraction from S. coelicolor wild-type cells grown for 2 days under blue light illumination. The spots identified as β-carotene (Rf = 0.54) (b) and isorenieratene (Rf = 0.24) (i) by HPLC analysis are indicated.
Subsequently, we found that the pigment production also occurs in wild-type S. coelicolor. Spectroscopic analysis of the carotenoid fraction extracted from the wild-type cells showed that the strain accumulates the pigment upon cultivation under light conditions, while it produced no yellow pigment under dark conditions (Fig. 1B). Marked production of the pigment occurred after 2 days of growth under illuminated conditions. The production profile in the wild-type strain was the same as that in the antibiotic-deficient strain described above. This indicated that regulation of light-induced carotenoid production is independent of the effect of the introduction of pKM284. We further examined carotenoid production in the wild-type strain.
S. coelicolor produced carotenoids when illuminated with blue light (λ = 400 to 460 nm) but not when illuminated with red light (λ = 600 to 700 nm). Thin-layer chromatography analysis of the carotenoid fraction extracted from the wild-type cells illuminated with blue light revealed the presence of at least five yellow pigment spots that showed different Rf values (Fig. 1C). Among these spots, two were identified as β-carotene and isorenieratene by HPLC analysis with standard controls.
Carotenoid biosynthesis gene cluster of S. coelicolor A3(2).
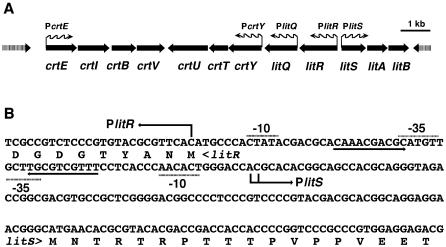
Figure 2A shows the carotenoid biosynthesis gene cluster and its flanking regions found in the genome sequence of S. coelicolor A3(2) (http://www.sanger.ac.uk/Projects/S_coelicolor) (5). The biosynthesis gene cluster consists of two convergent operon structures, crtEIBV and crtYTU. The gene organization is identical to that of the crt gene cluster in S. griseus (22). The crt gene cluster is also present in the genome of Streptomyces avermitilis (http://avermitilis.ls.kitasato-u.ac.jp) (14), which has the two operons in divergent orientations. The short distance between the crt genes suggests that transcription of the crt biosynthesis gene cluster is directed by the promoters preceding crtE and crtY.
FIG. 2.
Carotenoid biosynthesis gene cluster of S. coelicolor A3(2). (A) Schematic view of crt and the adjacent lit gene cluster. Wavy arrows indicate the orientations of the major transcripts. (B) Nucleotide sequence of the bidirectional promoter region between litR and litS. The N-terminal amino acid sequences of LitR and LitS are also shown. The transcriptional initiation sites determined by S1 mapping are indicated by bent arrows. An inverted repeat region is indicated by convergent arrows. Regions corresponding to the −35 and −10 sites are also indicated.
The S. coelicolor A3(2) crt gene cluster is flanked by a putative regulatory region that consists of two divergent operons, litRQ and litSAB (for light-induced transcription) (Fig. 2A). LitR (35 kDa) and LitS (22 kDa) are a MerR family transcriptional regulator and an RNA polymerase ECF sigma factor, respectively. LitA (23 kDa) is a possible lipoprotein. LitB (15 kDa) shows weak similarity to proteins known as anti-sigma factors, such as RsrR of S. coelicolor. LitQ (44 kDa) is a possible oxidoreductase. The lit cluster minus litAB is also present in the S. avermitilis genome but at a different locus from the crt cluster.
Inactivation of crt and lit genes and overexpression of litS.
We generated crt and lit gene mutants in order to examine the roles of the genes in carotenogenesis in S. coelicolor A3(2). All mutants except the litS mutant have an insertion of a kanamycin resistance gene, which is oriented so as not to cause polar effects on expression of other crt and lit genes except litR (see Materials and Methods). The crtEIBV deletion mutant did not produce the yellow pigments, which confirmed that the pigments are carotenoids synthesized by the crt gene cluster (Fig. 1B). Inactivation of litS by markerless construction also abolished the ability to produce carotenoids. This strongly suggested that the sigma factor gene is essential for the transcription of the crt biosynthesis gene cluster. Meanwhile, litAB and litB inactivation did not affect carotenoid production. Carotenoid production in the litS mutant was restored by the introduction of a single copy of the intact litS gene on an integration plasmid, pIJ8660 (18).
We were unsuccessful in isolating markerless litR and litQ disruptants. In both genes, the single-crossover segregant that carries both an intact and a mutated coding sequence was successfully obtained; however, the double crossover, which removes one of the alleles, generated only revertants that exhibited the wild-type genotype. We also failed to obtain the mutants even when the cells were cultured in the dark. On the other hand, we could obtain a litR mutant by replacing the wild-type litR allele with a mutated construct carrying a kanamycin resistance gene in the middle of the litR coding sequence, in the direction opposite to that of litR. The mutant was defective in carotenoid production, while its growth, colony morphology, and antibiotic production were unaffected. Carotenoid production in this mutant was not restored when a single copy of an intact litR gene on a plasmid was introduced.
We also examined the carotenoid production phenotype resulting from litS and litR overexpression. The wild-type strain, which harbors a high-copy-number plasmid that carries litS downstream of the mel promoter (see Materials and Methods), produced carotenoids independent of illumination at the same level as the wild type cultured with illumination. The introduction of the litS expression plasmid also resulted in light-independent carotenoid production in the litR mutant. On the other hand, introduction of a litR expression plasmid that had a construction similar to that of litS did not affect the light-dependent carotenoid production in the wild type or the carotenoid-deficient phenotype of any mutant strain.
S1 protection analysis.
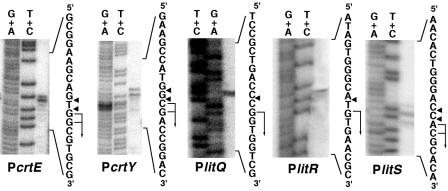
We examined the transcriptional activities of promoters preceding crtE (PcrtE), crtY (PcrtB), litQ (PlitQ), litR (PlitR), and litS (PlitS). High-resolution S1 protection analysis assigned a single transcriptional start site in each promoter (Fig. 3). It was noteworthy that the start site for PlitR was the first G residue of the translational initiation codon (GTG). The bidirectional promoter in the intergenic region between litR and litS had a symmetric structure centered on an inverted repeat that overlaps the −35 sequences of both promoters (Fig. 2B).
FIG. 3.
Determination of transcriptional start sites by high-resolution S1 protection assay. Maxam-Gilbert sequencing ladders (G+A and T+C reactions) were generated from the same 32P-labeled probe DNA fragments used in each S1 protection reaction. The positions of the S1-protected fragments are shown by the arrowheads, and the transcriptional initiation sites are assigned to the residues indicated by the bent arrows.
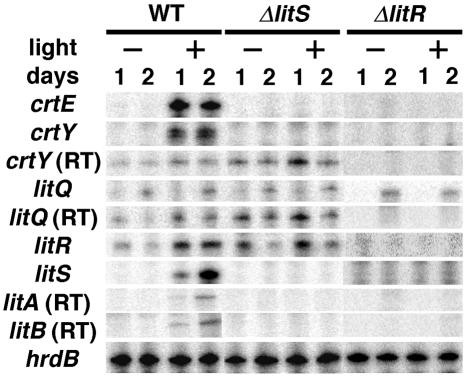
Quantification of transcripts by low-resolution S1 mapping showed that the majority of the promoters were light dependent (Fig. 4). In the wild-type strain, PcrtE, PcrtY, and PlitS activities were detected only under the light conditions. Meanwhile, PlitQ and PlitR showed activities under both dark and light conditions. PlitQ activity was relatively low and independent of illumination. PlitR showed lower activity under dark conditions than under light conditions. The probes for PcrtY and PlitQ also detected readthrough transcripts derived from PlitR (Fig. 2A). The probes for PlitA and PlitB detected only the readthrough transcript from PlitS, which indicates that litAB is cotranscribed with litS.
FIG. 4.
Quantification of promoter activities by low-resolution S1 protection assay. The promoters preceding the coding sequences listed at the left were examined for their activities in the wild type (WT) and in the S. coelicolor A3(2) litS (ΔlitS) and litR (ΔlitR) mutants. RNA was isolated from cells cultured for 2 days under dark (−) or light (+) conditions. For crtY, litQ, litA, and litB, the signals derived from the readthrough transcripts (RT) are also shown. Transcription of hrdB was analyzed to confirm the quality and quantity of RNA.
We also examined promoter activities in the litS and litR mutants (Fig. 4). In the markerless litS mutant, the activities of PcrtE, PcrtY, and PlitS were completely abolished. This suggested that PcrtE, PcrtY, and PlitS depend on σLitS. Comparison of the putative σLitS-dependent promoters revealed the presence of a possible consensus sequence, GCAT(G/C)-(16 or 17 bp)-C(G/C)(G/C)AA(G/C) at the −35 and −10 regions (Fig. 5). On the other hand, litS inactivation did not affect the activities of PlitR and PlitQ. The same analysis with the kanamycin-resistant litR mutant revealed that all promoter activities except that of PlitQ were completely abolished by the mutation. The S1 analysis with the probe for PlitS in the litR mutant detected a readthrough transcript derived from the promoter of the kanamycin resistance gene cassette, which had been inserted in the coding sequence of litR in the opposite orientation (data not shown). All activities of the lit and crt promoters in the litAB mutant were the same as those in the wild-type strain (data not shown).
FIG. 5.
Sequence alignment of the σLitS-dependent promoters. The solid boxes show a possible consensus sequence for recognition by EσLitS. Transcription initiation sites are underlined.
In vitro runoff transcription analysis.
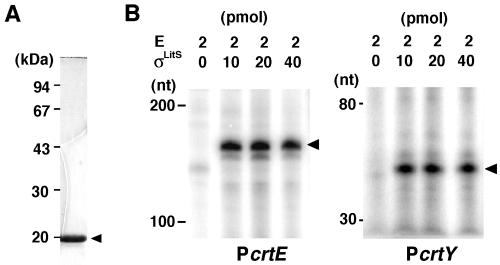
We performed an in vitro runoff transcriptional assay in order to examine the dependence of PcrtE, PcrtY, and PlitS on σLitS. Recombinant σLitS protein was prepared by use of an E. coli expression-purification system (Fig. 6A). As shown in Fig. 6B, the RNA polymerase holocomplex containing the purified σLitS protein generated specific transcripts on the probe DNA fragments for both PcrtE and PcrtY. However, the holoenzyme did not generate a specific transcript on the probe DNA for the PlitS region, which led us to assume that the transcriptional initiation at PlitS requires an additional transcription factor, possibly LitR. Thus, we prepared purified LitR protein and assessed its effect on in vitro transcription by EσLitS on a PlitS template; however, we failed to detect any specific transcript. In a gel mobility shift assay, the recombinant LitR protein did not bind DNA fragments containing PlitS.
FIG. 6.
In vitro runoff transcription by EσLitS. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the σLitS protein expressed and purified by use of an E. coli host-vector system. (B) Polyacrylamide gel electrophoresis of σLitS-specific transcripts. The indicated amounts of an E. coli RNA polymerase core enzyme (E) and the recombinant σLitS protein were added to the reaction mixture with the promoter DNA fragments to generate transcripts specific for crtE (PcrtE) and crtY (PcrtY). Arrowheads indicate the specific transcripts. nt, nucleotides.
DISCUSSION
In Streptomyces, carotenoid production is a widespread metabolic activity, which occurs in a constitutive, light-dependent, or cryptic manner (20). This implies the presence of a certain diversity in the molecular mechanisms of carotenoid production in this group of bacteria. To date, genetic studies on carotenoid production in Streptomyces have been described only for Streptomyces setonii (15) and S. griseus (22), both of which show cryptic carotenogenesis. Carotenoid production in these organisms is induced by an increased copy number of a gene that encodes a stress-response sigma factor, σCrtS. This suggests that this sigma factor is involved in the transcription of carotenoid biosynthesis genes in these species. On the other hand, S. coelicolor A3(2), examined in this study, does not retain the crtS homolog in its genome, and it was shown that an ECF sigma factor, σLitS, responsible for light-induced carotenoid production is present. The litQRS gene cluster is conserved in S. avermitilis, which also produces carotenoids in a light-dependent manner (H. Ikeda, personal communication). The presence of lit genes in the two phylogenetically divergent species raises the possibility that the regulatory mechanism is common to light-induced carotenoid production in this group of bacteria.
In terms of the molecular mechanism of light-dependent carotenogenesis, the best-studied nonphototrophic bacterium is M. xanthus, in which an ECF sigma factor is involved in the regulation of carotenoid production (8, 10, 23, 29). In M. xanthus, the light-inducible ECF sigma factor σCarQ directs transcription of the carQRS operon. Thus, expressed CarS acts as a positive regulator of the transcription of the carotenoid biosynthesis gene cluster. This transcription occurs through sequestration of a transcriptional repressor protein, CarA, which binds to the major promoter of the biosynthesis gene cluster to block transcription under dark conditions. The activity of σCarQ is negatively regulated by the adjacent anti-sigma factor CarR. CarR is a membrane protein that is unstable under illuminated conditions, and it is assumed to release active σCarQ upon illumination. Related studies have shown the presence of additional regulatory components involved in light-induced transcriptional control in M. xanthus (10).
The regulatory mechanism in S. coelicolor appears to be simple in comparison with the complex system in M. xanthus. S. coelicolor A3(2) LitS shows 21.3% amino acid sequence identity to M. xanthus σCarQ. However, the S. coelicolor genome does not contain homologous genes for CarR, CarS, or CarA of M. xanthus. While there is no in vitro evidence for transcription of crt genes by σCarQ in M. xanthus (8), S. coelicolor σLitS transcribes the crt operons. Such marked diversity in control mechanisms may reflect the ecological niches of those organisms.
The transcriptional analyses showed that light-induced transcriptional control underlies the photodependent carotenoid production in S. coelicolor A3(2). The S1 mapping result for the litS mutant strongly suggested that σLitS directs its own transcription. This indicates that litS comprises a positive feedback circuit, which may enable signal amplification that facilitates crt transcription upon receiving light. However, the σLitS-dependent transcription from the litS promoter was not reproduced in the in vitro transcriptional assay, while transcription from the crt promoters was successfully demonstrated. We speculate that transcription from the litS promoter requires an additional element(s), such as a transcriptional activator protein. The symmetric and compact structure of the bidirectional promoter region between litS and litR suggests that a photodependent regulator simultaneously controls the initiation of transcription in both directions. This may account for the photodependence of the litR promoter. The promoter region is highly conserved in the S. avermitilis counterpart, which suggests that an identical mechanism controls the light-dependent carotenoid production in this organism.
We were unsuccessful in isolating markerless mutants for litR and litQ, probably due to the lethal effect of the mutation. The litR mutant isolated in this study carries an aphII cassette that has a polar effect in the direction of litS and downstream genes. At present, we cannot clearly explain why the mutant construction did not affect viability. One possibility is that the constitutive readthrough transcription from the aphII promoter in the orientation toward litS compensates for the litR deficiency. Since polar transcription should confer low-level LitSAB expression, some functions of these proteins may be related to suppression of the lethality caused by litR inactivation. The suppression mechanism may also be related to the failure in the genetic complementation of the litR mutant, although we have no convincing working model for this mechanism.
Transcriptional analysis of the litR mutant demonstrated that all promoter activities, except litQ, are abolished in the mutant background. This result leads to a simple hypothesis for the light induction of litS: LitR acts as an essential transcriptional activator of the litS promoter under light conditions. This accounts for the effect of litR depletion on the promoter activities and carotenoid productivity. The lack of litR promoter activity in the litR mutant implies that LitR also acts as a positive regulator of its own promoter and thus comprises a positive feedback loop. Although this proposition appears to be justified, we believe it should be supported further by additional experimental results, particularly those using different genetic backgrounds. This is because we cannot overlook the possibility of the presence of an artifact caused by the polar effect in the litR mutant. Meanwhile, the effect of the high copy number of litS, which resulted in constitutive carotenoid production in the litR mutant, greatly supports the idea that litS is under litR control, such that litS overexpression can suppress the litR deficiency.
LitR shows an end-to-end similarity to the MerR-type transcriptional regulator, which usually acts as a transcriptional activator by binding to an inverted repeat structure localized in the vicinity of the −35 region (7). The compact structure of the bidirectional promoter region between litR and litS appears to meet the condition for the action of this type of transcriptional regulator. Usually, MerR family regulators acquire DNA-binding ability by ligand binding (7). It is possible that the LitR recombinant protein prepared in this study requires the supply of a specific ligand in order to bind the intergenic promoter region between litS and litR in the in vitro DNA-binding assay. If this is the case, identification of the ligand molecule should serve as an important clue for uncovering the photodependent regulatory mechanism in S. coelicolor. We speculate that litQ may also be related to the control system, based both on the conserved features in two different Streptomyces species and on the lethality of its inactivation, as shown by litR. The amino acid sequence similarity search suggested that LitQ is an oxidoreductase, and its function may be related to the link between illumination and activation of the LitR protein. Studies on the function of Lit proteins will reveal a novel light-responding control mechanism in this group of bacteria.
Acknowledgments
We thank David Hodgson, Mark Buttner, Yasuo Ohnishi, and Haruo Ikeda for helpful discussion. We also thank Tomoyoshi Akashi for technical assistance and Helen Kieser for providing S. coelicolor cosmids.
This study was supported by the 21st Century COE Program of MEXT, Japan. H.T. was supported by a JSPS fellowship grant.
REFERENCES
- 1.Armstrong, G. A., M. Alberti, and J. E. Hearst. 1990. Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc. Natl. Acad. Sci. USA 87:9975-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, G. A., and J. E. Hearst. 1996. Carotenoids. 2. Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 10:228-237. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, G. A., A. Schmidt, G. Sandmann, and J. E. Hearst. 1990. Genetic and biochemical characterization of carotenoid biosynthesis mutants of Rhodobacter capsulatus. J. Biol. Chem. 265:8329-8338. [PubMed] [Google Scholar]
- 4.Beck, E., G. Ludwig, E. A. Auerswald, B. Reiss, and H. Schaller. 1982. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene 19:327-336. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 6.Botella, J. A., F. J. Murillo, and R. Ruiz-Vazquez. 1995. A cluster of structural and regulatory genes for light-induced carotenogenesis in Myxococcus xanthus. Eur. J. Biochem. 233:238-248. [DOI] [PubMed] [Google Scholar]
- 7.Brown, N. L., J. V. Stoyanov, S. P. Kidd, and J. L. Hobman. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145-163. [DOI] [PubMed] [Google Scholar]
- 8.Browning, D. F., D. E. Whitworth, and D. A. Hodgson. 2003. Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol. Microbiol. 48:237-251. [DOI] [PubMed] [Google Scholar]
- 9.Burchard, R. P., and M. Dworkin. 1966. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J. Bacteriol. 91:535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontes, M., L. Galbis-Martinez, and F. J. Murillo. 2003. A novel regulatory gene for light-induced carotenoid synthesis in the bacterium Myxococcus xanthus. Mol. Microbiol. 47:561-571. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin, T. W. 1980. The biochemistry of the carotenoids, vol. 1. Chapman and Hall, Ltd., London, United Kingdom.
- 12.Grogan, D. W. 1989. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 171:6710-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 100:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. Omura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526-531. [DOI] [PubMed] [Google Scholar]
- 15.Kato, F., T. Hino, A. Nakaji, M. Tanaka, and Y. Koyama. 1995. Carotenoid synthesis in Streptomyces setonii ISP5395 is induced by the gene crtS, whose product is similar to a sigma factor. Mol. Gen. Genet. 247:387-390. [DOI] [PubMed] [Google Scholar]
- 16.Katz, E., C. J. Thompson, and D. A. Hopwood. 1983. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J. Gen. Microbiol. 129:2703-2714. [DOI] [PubMed] [Google Scholar]
- 17.Kelemen, G. H., P. H. Viollier, J. Tenor, L. Marri, M. J. Buttner, and C. J. Thompson. 2001. A connection between stress and development in the multicellular prokaryote Streptomyces coelicolor A3(2). Mol. Microbiol. 40:804-814. [DOI] [PubMed] [Google Scholar]
- 18.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 19.Komatsu, M., Y. Kuwahara, A. Hiroishi, K. Hosono, T. Beppu, and K. Ueda. 2003. Cloning of the conserved regulatory operon by its aerial mycelium-inducing activity in an amfR mutant of Streptomyces griseus. Gene 306:79-89. [DOI] [PubMed] [Google Scholar]
- 20.Koyama, Y., F. Kato, and Y. Yazawa. 1976. Effect of light on the pigmentation of bacteria in Actinomycetales. p. 65-85. In T. Arai (ed.), Actinomycetales, the boundary microorganisms. Toppan, Tokyo, Japan.
- 21.Krugel, H., P. Krubasik, K. Weber, H. P. Saluz, and G. Sandmann. 1999. Functional analysis of genes from Streptomyces griseus involved in the synthesis of isorenieratene, a carotenoid with aromatic end groups, revealed a novel type of carotenoid desaturase. Biochim. Biophys. Acta 1439:57-64. [DOI] [PubMed] [Google Scholar]
- 22.Lee, H. S., Y. Ohnishi, and S. Horinouchi. 2001. A sigmaB-like factor responsible for carotenoid biosynthesis in Streptomyces griseus. J. Mol. Microbiol. Biotechnol. 3:95-101. [PubMed] [Google Scholar]
- 23.Lopez-Rubio, J. J., M. Elias-Arnanz, S. Padmanabhan, and F. J. Murillo. 2002. A repressor-antirepressor pair links two loci controlling light-induced carotenogenesis in Myxococcus xanthus. J. Biol. Chem. 277:7262-7270. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Schumann, G., H. Nurnberger, G. Sandmann, and H. Krugel. 1996. Activation and analysis of cryptic crt genes for carotenoid biosynthesis from Streptomyces griseus. Mol. Gen. Genet. 252:658-666. [DOI] [PubMed] [Google Scholar]
- 26.Takano, H., K. Hosono, T. Beppu, and K. Ueda. 2003. Involvement of σH and related sigma factors in glucose-dependent initiation of morphological and physiological development of Streptomyces griseus. Gene 320:127-135. [DOI] [PubMed] [Google Scholar]
- 27.Ueda, K., K. Matsuda, H. Takano, and T. Beppu. 1999. A putative regulatory element for carbon-source-dependent differentiation in Streptomyces griseus. Microbiology 145:2265-2271. [DOI] [PubMed] [Google Scholar]
- 28.Weeks, O. B., and R. J. Garner. 1967. Biosynthesis of carotenoids in Flavobacterium dehydrogenans Arnaudi. Arch. Biochem. Biophys. 121:35-49. [DOI] [PubMed] [Google Scholar]
- 29.Whitworth, D. E., and D. A. Hodgson. 2001. Light-induced carotenogenesis in Myxococcus xanthus: evidence that CarS acts as an anti-repressor of CarA. Mol. Microbiol. 42:809-819. [DOI] [PubMed] [Google Scholar]
- 30.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]