Abstract
Transcription of the repressible acid phosphatase gene (KIPHO5) in Kluyveromyces lactis is strongly regulated in response to the level of inorganic phosphate (Pi) present in the growth medium. We have begun a study of the promoter region of this gene in order to identify sequences involved in the phosphate control of KIPHO5 expression and to design new expression-secretion systems in K. lactis. Deletion analysis and directed mutagenesis revealed two major identical upstream activating sequences (UAS) CACGTG at positions −430 (UAS1) and −192 (UAS2) relative to the ATG initiation codon. These sequences are identical to those described for Saccharomyces cerevisiae for the binding of Pho4p. Deletion or directed mutagenesis of either one or both UAS reduce KIPHO5 expression by the same amount (approximately 80%). When fused to the coding region of trout growth hormone cDNA (tGH-II), the promoter and signal peptide-encoding region of the phosphate-repressible KIPHO5 gene drives the expression of this gene and the secretion of the tGHII protein. Synthesis of tGHIIp in K. lactis transformants carrying this construct was found to be regulated by the Pi present in the medium; derepression of heterologous protein expression can therefore be achieved by lowering the Pi concentration.
Kluyveromyces lactis has recently become an attractive microbial host for the expression of foreign genes and protein secretion for several reasons, including (i) its food grade status, since K. lactis is present in various milk products it is accepted as “GRAS” (generally recognized as safe); (ii) its excellent fermentation characteristics (14); (iii) the existence of both episomal and integrative vectors (45); and (iv) its ability to secrete high-molecular-weight proteins (9, 14, 15, 34, 39).
The promoter and the secretory signal are key elements in all expression systems. Several Saccharomyces cerevisiae promoters, including UASgal/CYC1 (25), PGK (7, 14), PHO5 (6), and GAL7 (4), and secretory signals, including MFα (42), SUC2 (3), or those included in the heterologous protein (HSA [13], prepro-HSA [39]), have been used to generate heterologous protein secretion in K. lactis. Surprisingly, only three K. lactis promoters (GAL7 [35], LAC4 [13, 39], and ADH4 [11a]) and one secretory signal (the one for the killer toxin [14, 35]) have been used in this context. Recently, efficient expression and secretion of mouse α-amylase (under the 128-kDa precursor protein and in shuttle vectors with S. cerevisiae PHO5 and the PGK promoter and terminator sequences) into the culture medium have been described in K. lactis (40).
The availability of a variety of K. lactis-based expression systems is desirable both from a commercial standpoint and from a research standpoint. Accordingly, our laboratory has isolated several K. lactis-regulated genes. One of them is the repressible KIPHO5 gene that encodes a secreted acid phosphatase (APase [12]). The gene has all the features necessary for the basis of an alternative expression system for the secretion of heterologous proteins in K. lactis. The expression of KIPHO5 can be turned on by the simple and cheap procedure of lowering the Pi concentration in the medium (12).
Furthermore, to obtain deregulated strains of K. lactis in response to Pi, we took advantage of the fact that the KIPHO5 gene strongly resembles the ScPHO5 gene, whose promoter region has been extensively analyzed (36, 43), as has its secretory signal (1, 2, 17) and its use in heterologous protein production (7, 20). The present study reports on a functional analysis, using a combination of deletion and directed mutagenesis, of the KIPHO5 promoter. Three upstream activating sequence (UAS) elements, UAS1, UAS2, and UAS3, were located in the promoter. Deletions or nucleotide substitution in all of them show reduced transcription of KIPHO5. The aim of this study was to use the regulatory elements and the secretion signal of the secreted KIPho5p encoded by the repressible KIPHO5 gene of K. lactis to develop a regulated secretion system for heterologous proteins with trout growth hormone (coded by tGHII) as a model protein.
MATERIALS AND METHODS
Strains and media.
The Escherichia coli strains used for transformation and amplification of recombinant DNA were (i) HB101 F− hsdS20 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 (Smr) xyl-5 mtl-1 supE44 λ− (8); (ii) DH5α supE44 ΔlacU169(φ80 lacZ ΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 (18); and (iii) MV1190 Δ(lac-pro AB) thi supE Δ(sr1-recA)306::Tn10(Tetr) (F′: traD36 proAB lacIqZ ΔM15) (Bio-Rad). E. coli strains were grown in Luria-Bertani broth.
K. lactis 2359/152F (MATa metA1-1 ura3-20) (12) was used as the recipient strain in all of our experiments. The organism was maintained on YED medium (yeast extract, 1%; glucose, 1%; agar, 2%) slants. Yeast cultures were grown at 28°C in 1,000-ml Erlenmeyer flasks with 300 ml of medium in a gyratory shaker at 250 rpm on minimal liquid medium (MM) (0.7% yeast nitrogen base [Difco], 1% glucose [46]). Media were supplemented with uracil and methionine each at 50 μg · ml−1 as required. As derepression medium we used MM in which the phosphate concentration was lowered to 10 mg/liter (low Pi). The medium was buffered with 0.05 M citric acid-sodium citrate (pH 4.3).
Cell extracts.
Packed cells (approximately 18 mg, wet weight) were resuspended in citric buffer. Equal volumes of yeast cell suspension and glass beads (0.45 to 0.50 mm in diameter) were broken by mechanical shaking in a FastPrep FP120 homogenizer. This treatment disrupted >98% of the cells as judged by light microscope examination. The glass beads were removed by washing the broken-cell suspension. This fraction was called “total protein” (see Fig. 4) or cell extracts. The total amount of protein found in the culture medium was referred to as “extracellular protein.”
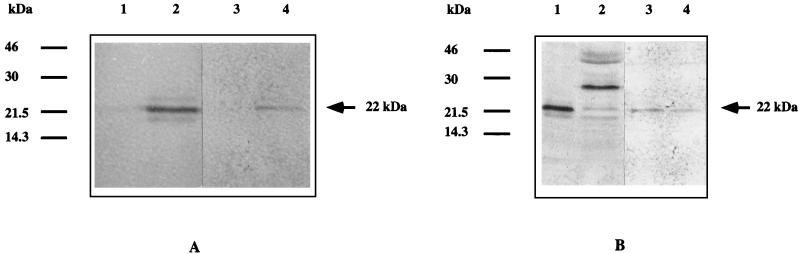
FIG. 4.
Expression of trout growth hormone (tGHII) in K. lactis. (A) Western blot analysis of total yeast protein (cell extract, lanes 1 and 2) and culture medium (lanes 3 and 4). K. lactis 2359/152F cells transformed with pEFKGHII were harvested after 8 h in MM control cultures (lanes 1 and 3) or after 8 h in low-Pi medium (derepression conditions, lanes 2 and 4). Then 50 μg (lanes 1 and 2) or 10 μg (lanes 3 and 4) of total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto nitrocellulose, and immunodetected with rabbit anti-tGHII polyclonal antibodies. (B) Western blot analyses of total yeast proteins (cell extract, lanes 1 and 2) and culture medium (lanes 3 and 4). Cells of K. lactis 2359/152F transformed with pEFKGHII (lanes 1 and 3) or pEtGHII (lanes 2 and 4) were harvested after 8 h of derepression in low-Pi medium and treated as described for panel A (lanes 1 and 2, 50 μg of protein; lane 3, 10 μg of protein; lane 4, 100 μg of protein).
Enzyme activity.
Acid phosphatase activity was assayed with p-nitrophenyl phosphate (PNPP) (Sigma Chemical Co., St. Louis, Mo.). The assay mixture was composed of 250 μl of enzyme sample, 125 μl of 0.36 M citric acid-sodium citrate buffer (pH 4.3), and 75 μl of 0.04 M PNPP. The reaction was initiated by the addition of the substrate and terminated by the addition of 750 μl of 0.1 M NaOH. One unit of activity was defined as the amount of enzyme which released 1 nmol of p-nitrophenol in 1 min at 30°C. β-Galactosidase was assayed by the method of Miller (26).
Yeast transformation was carried out by electroporation (38). For selection of transformants, cells were plated on MM agar (0.67% yeast nitrogen base without amino acids; 2% glucose; 1.5% agar, supplemented with methionine at 50 μg · ml−1). K. lactis vectors are shown in Fig. 1A.
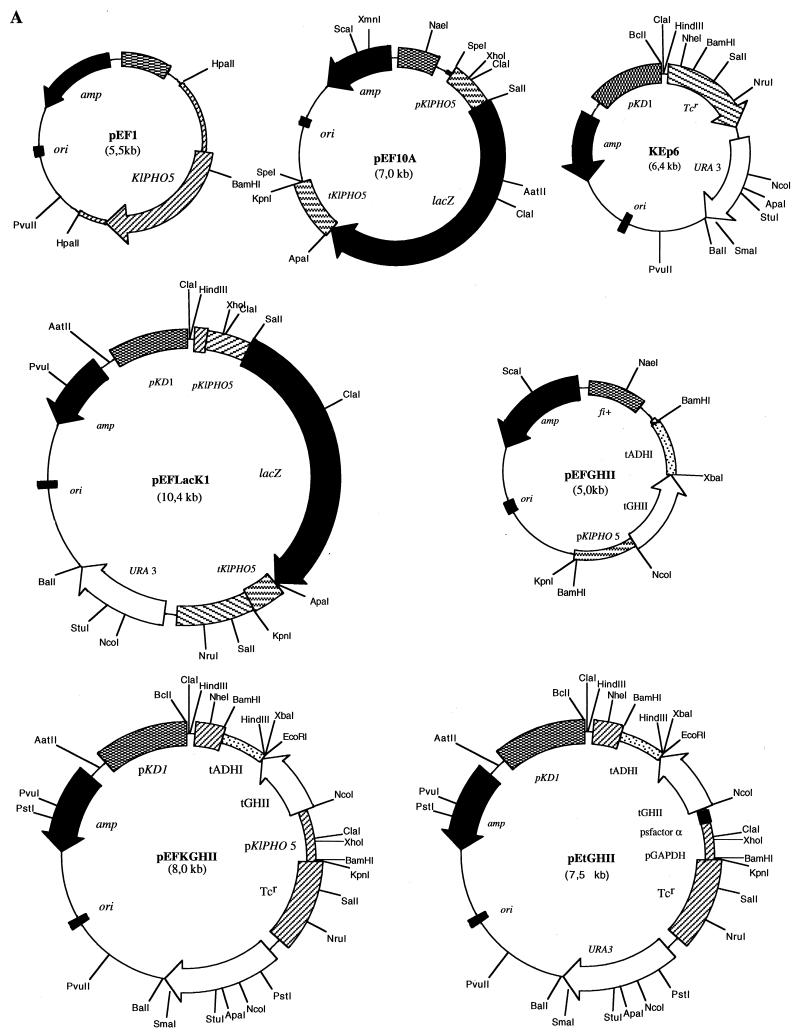
FIG. 1.
Construction of expression vectors. (A) The position of the signal peptide (SP) cleavage site in K. lactis APase encoded by KIPHO5 was predicted to be Ala16-Ala17 by the method of von Heijne (44) and confirmed by purification of mature APase and N-terminal sequencing (12). Plasmid pEF1, which contains the entire KIPHO5 gene of K. lactis, was used as template in PCR to isolate a 507-bp fragment comprising the KIPHO5 promoter and the first 17 codons of the KIPHO5 gene. After digestion with SalI this fragment was ligated to a 3,100-bp SalI-SalI fragment from pMC1871 (10) (Fig. 1B) containing the β-galactosidase gene. The KIPHO5 transcriptional termination signals (a 630-bp fragment [12]) were added 3′ to the SalI place of the KIPHO5 promoter β-galactosidase fusion, giving rise to plasmid pEF10A. (B and C) The whole 4,237-bp SpeI-SpeI fragment was inserted into the NheI site of the episomal plasmid KEp6 (45), yielding pEFLacK1. pEFGHII resulted from inserting a 504-bp fragment comprising the KIPHO5 promoter and the first 16 codons of the KIPHO5 gene with the NcoI-BamHI fragment containing the tGHII gene and the ADHI terminator (see Fig. 1A, plasmid pEtGHII). The whole 1,686-bp BamHI-BamHI fragment was inserted in the BamHI site of KEp6, giving rise to pEFKGHII (Fig. 1A).
Protein assays.
Protein content was determined colorimetrically by the method of Lowry et al. (24). Bovine serum albumin was used as a standard.
Endo H treatment.
Samples were incubated with endo-β-N-acetylglucosaminidase H (Endo H) for the desired times at 37°C in 0.05 M acetic acid-sodium buffer (pH 5.6) containing 0.001 M phenylmethylsulfonyl fluoride and 10 μM pepstatin.
Electrophoresis, electroblotting, and immunological detection.
Slab gel electrophoresis was performed essentially as described by Laemmli (23). Electrotransfer of proteins to nitrocellulose membranes and immunological reactions were performed as described previously (11, 41).
Protein stains.
Gel slabs were stained for protein by a silver staining method (27).
DNA manipulations.
Total DNA from K. lactis was prepared as described for filamentous fungi (32). Restriction enzyme digestions and DNA ligations were performed according to the recommendations of the manufacturers. Isolation of plasmid DNA from E. coli was performed by standard procedures (37).
PCR amplifications.
PCR experiments were performing with Taq DNA polymerase as recommended by the supplier (Perkin-Elmer Cetus Corp.). The PCR conditions to amplify K. lactis DNA were as follows: 10 ng of the selected plasmid was mixed with 50 pmol of each primer in a final reaction volume of 50 μl and subjected to 30 amplification cycles (95°C for 1 min, 42°C for 1 min, and 72°C for 1 min).
Sequence analysis of PCR fragment.
The DNA restriction fragment harboring the corresponding KIPHO5 promoter fused to the β-galactosidase was subcloned into the pBluescript plasmids (SK+ and KS+; Stratagene), and a nested set of closely spaced deletions was created by using exonuclease III (19, 37). All deletion endpoints, site-directed mutagenesis, and the structures of each fusion plasmid were verified by DNA sequencing and restriction mapping. The products of the sequencing reactions were resolved on buffered gradient polyacrylamide-urea sequencing gels (5) and exposed to Kodak XAR-5 X-ray film. DNA and protein sequences were analyzed with the DNASIS and PROSIS programs (Pharmacia-LKB and Hitachi), respectively.
RESULTS AND DISCUSSION
Basis of analysis of deletions and mapping cis-acting regulatory sequences in the KIPHO5 flanking region.
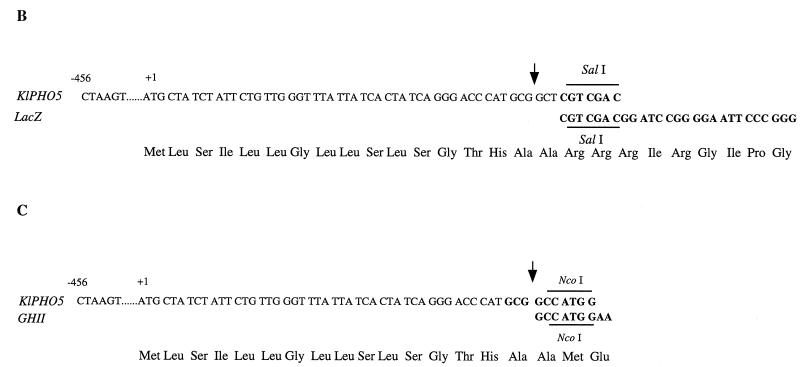
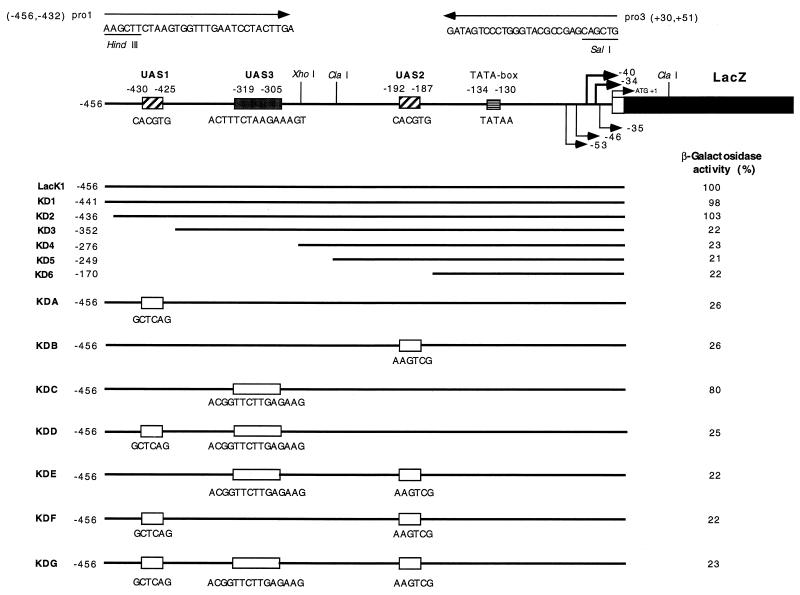
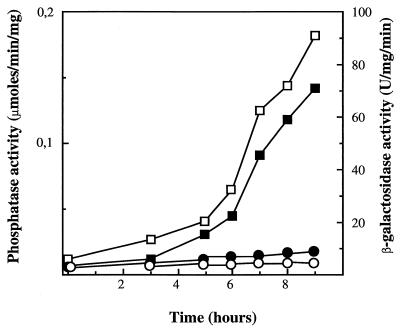
To mark important elements in the KIPHO5 promoter after its comparison with the S. cerevisiae PHO5 promoter (12, 36), we defined a 456-bp upstream region that contained all the putative cis-acting regulatory elements. Using complementary oligonucleotides (pro1 and pro3 [see Fig. 3]), we PCR-amplified with plasmid pEF1 as template (Fig. 1A) a 507-bp fragment comprising the whole KIPHO5 promoter and all 17 amino acids of the signal peptide, extended for HindIII (5′) and SalI (3′) sites (the fragment was sequenced in order to ascertain the correct PCR amplification). This fragment was fused with the E. coli lacZ gene, coming from plasmid pMC1871, as the reporter (10) (Fig. 1B). An identical procedure was carried out to amplify a 630-bp fragment of the KIPHO5 terminator with oligonucleotides ter 1 (AGTAACAAGTAAGTCTCATACCGA) and ter 3 (AAGATACCGGGTACCACTAGTATCGATCTTATATTTTGCTCT) to give plasmid pEF10A (Fig. 1A). Insertion of this SpeI-SpeI fragment into the NheI fragment of KEp6 gave rise to pEFLacK1 (Fig. 1A). We transformed a K. lactis ura3 strain previously constructed by us (12) with pEFLacK1 (see Fig. 1A). On high-phosphate medium, β-galactosidase and acid phosphatase activity levels remained constant regardless of the incubation time (Fig. 2). On low-phosphate medium, acid phosphatase and β-galactosidase were derepressed (Fig. 2). The promoter region of KIPHO5 was then screened for functionally important DNA sequences by the construction of a series of deletion mutants (Fig. 3). The effects of the deletions were assayed by measuring β-galactosidase activity on the Ura+ transformants in low- and high-phosphate media. Our results pointed to an initial regulatory region located between positions −436 and −352. Deletion of this region elicited a decrease of 70 to 85% of total β-galactosidase activity. Further deletions did not produce any further decrease in β-galactosidase activity (KD4, KD5, and KD6; Fig. 3). By comparison with the S. cerevisiae PHO5 promoter, we identified two CACGTG upstream hexanucleotide sequences at positions −430 and −192 in the KIPHO5 promoter.
FIG. 3.
The KIPHO5 promoter, deletion analyses, and site-directed mutagenesis derivatives. The restriction map shows 456 bp of the wild-type promoter. mRNA starts are indicated by arrows; the TATA box and ATG are marked. A white box indicates the 16 amino acid signal peptide. The whole sequence has been published previously (12). Insertion of mutated oligomers into UAS1, UAS2, or UAS3 was carried out by PCR (22) with pEFLacK1 as the template and the oligonucleotides shown in the figure. The primers pro1 and pro3 are shown. The names of the plasmids bearing each construction are on the left. The values of β-galactosidase measured under derepression conditions are on the right (see Fig. 2). The residual activities of K. lactis 2359/152F bearing each plasmid is given as a percentage of the activity carried by the control pEFLacK1.
FIG. 2.
Derepression kinetics of KIPHO5 and β-galactosidase activity in K. lactis cells (strain 2359/152F transformed with plasmid pEFLacK1) starved for Pi. Exponentially growing cells in MM were resuspended in low-Pi medium. APase and β-galactosidase activities were assayed in washed cells. β-Galactosidase activity: □, low-Pi; ○, MM. APase activity: ▪, low-Pi; •, MM.
Insertion of synthetic oligomers with modifications in the UAS.
To confirm the putative UAS regulatory elements, we carried out directed mutagenesis. We designed oligomers in which the nucleotide sequence of each UAS had been changed (see Fig. 3). After PCR amplification and subcloning of the corresponding fragments, all possible combinations were tested; the results are shown in Fig. 3. Changes in the two previously described hexanucleotides (UAS1 at position −430 and UAS2 at position −192) dramatically decreased the level of β-galactosidase activity under derepression conditions (low-Pi medium). The decrease in β-galactosidase activity was similar to that obtained by deletion of the 276-bp fragment (KD6; Fig. 3).
Our results suggested (i) that the KIPHO5 promoter carries two essential cis-acting positive elements, a distal one (UAS1) located between positions −430 and −425 and a proximal one (UAS2) located between positions −192 and −187, and (ii) that cooperation between both UAS is required for full expression of the KIPHO5 gene.
In S. cerevisiae, genetic evidence has suggested that Pho2p and Pho4p bind to or interact with UAS in the promoter region of the phosphate-repressible acid phosphatase gene PHO5 (for a review, see reference 29). Conflicting data about the binding sites of Pho4p and Pho2p in the S. cerevisiae PHO5 promoter (4, 28, 36, 43) and for the Penicillium chrysogenum phoA gene (16) have been reported. The results shown in Fig. 3 are in agreement with those described by Rudolph and Hinnen (36) and Vogel et al. (43). We unequivocally identified the CACGTG motif as being essential for the derepression of acid phosphatase activity in K. lactis. The 6-bp motif was flanked by an A residue (UAS1) or a G residue (UAS2) at the 5′ end and by CA or TA residues at the 3′ end, respectively. Since no clear correlation between the flanking sequences of either UAS, nor between those described for S. cerevisiae, could be established, we propose that only the hexanucleotide sequence would be relevant for Pho4p binding to the promoter.
Another region, ACTTTCTAAGAAAGT (UAS3), located at −319 of the ATG between UAS1 and UAS2, also reduces KIPHO5 expression (by approximately 20%; see KDC, Fig. 3) and appeared to be involved in the regulation of KIPHO5 expression. This region could be equivalent to the Pho2p binding site described in the ScPHO5 promoter region (19, 43). Our results, however, do not permit us to assign a defined role to this region. No additive effects on β-galactosidase expression were obtained by combining mutations in this region with mutations in UAS1 (KDD), UAS2 (KDE), or both (KDG) (Fig. 3).
Analysis of the promoter region of the Pichia pastoris acid phosphatase (30) did not reveal any equivalent hexanucleotide (CACGTG/T) over 313 bp upstream from the initial ATG. Whether the identity between the cis-acting elements in the ScPHO5 and KIPHO5 promoters might be due to the close phylogenetic relationships between both yeasts (31) or whether these UAS are essential regulatory elements in all fungal acid phosphatases remains to be elucidated.
Detection and regulation by phosphate concentrations of tGHII secretion in K. lactis.
Using the 456-bp promoter and the 51 bases corresponding to the 17 amino acids of the signal peptide of the KIPHO5, we accomplished an in-frame fusion with the trout growth hormone tGH-II gene (33) (Fig. 1C) to obtain plasmid pEFGHII (Fig. 1A). A BamHI-BamHI fragment of 1,686 kb was inserted into the BamHI site of plasmid KEp6 (Fig. 1A), yielding pEFKGHII (Fig. 1A). We transformed K. lactis 2353/152F with pEtGHII (Fig. 1A), which was kindly supplied by Eurogentec, and with our construction (pEFKGHII) selected eight Ura+ transformants from each transformation that were further analyzed. Liquid cultures were grown in MM, and cells in the exponential growth phase were collected and resuspended at 108 cells/ml in MM (Pi concentration, 1 g/liter [control culture]) and in low-Pi medium (Pi concentration, 0.01 g/liter [derepression medium]). The levels of tGHIIp in cell extracts and in the culture medium were determined by immunoassay. Figure 4A (lanes 1 and 3) shows that under repression conditions the amount of tGHIIp was undetectable. However, under derepression conditions trout growth hormone appeared both in cell extracts (Fig. 4A, lane 2) and in the culture medium (Fig. 4A, lane 4). The maximum amount of hormone was obtained after 8 to 10 h of incubation under derepression conditions, a finding that is in agreement with our previous results for obtaining the maximal amount of KIPho5p (12). All eight transformants assayed showed the same behavior. Trout growth hormone has two potential N-glycosylation sites (33), but the addition of tunicamycin to cell cultures or the treatment of cell extracts or supernatants with Endo H did not produce any alteration in the size of the band, suggesting that the protein was not glycosylated in K. lactis.
To compare the efficiency of our vector expression with that achieved with a constitutive promoter (the one from glyceraldehyde phosphate dehydrogenase, plasmid pEtGHII) (Fig. 1A), we ran parallel experiments with both K. lactis transformants. The results are shown in Fig. 4B. Whereas in cell extracts, with our construction, only one band of the expected size of 22 kDa appeared (Fig. 4B, lane 1), in cell extracts of K. lactis 2359/152F transformed with pEtGHII several bands were visible (Fig. 4B, lane 2). Whether the band that migrated more slowly (with an apparent molecular size of 27 kDa) would correspond to aggregates or whether it would correspond to a peptide containing uncleaved α-factor signal peptide remains to be elucidated.
Both types of expression vectors secrete the tGHII protein into the culture medium efficiently (Fig. 4B, lanes 3 and 4). We quantified the total amount tGHIIp produced with both vectors. Under our most favorable conditions for acid phosphatase derepression (pH 4.3, 8 h of growth in low-Pi medium), we were able to produce about 20 times more tGHIIp (referred to as total cell or protein content) in the culture medium.
Our results show that it is possible to obtain high tGHIIp yields and that even though further studies are needed to purify the hormone and to test its biological activity, the useful regulatory properties of the KIPHO5 promoter and the APase signal peptide may be harnessed for production purposes.
ACKNOWLEDGMENTS
The authors wish to thank Eurogentec SA for providing plasmid pEtGHII and anti-tGHII rabbit antiserum.
This work was partially supported by grants from the CICYT (BIO92-0304 and BIO95-0518) and EU (BIO4-CT96-0003).
REFERENCES
- 1.Arima K, Oshima T, Kubota I, Nakamura N, Mizunaga T, Toh-e A. The nucleotide sequence of the yeast PHO5 gene: a putative precursor of repressible acid phosphatase contains a signal peptide. Nucleic Acids Res. 1983;11:1657–1673. doi: 10.1093/nar/11.6.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajwa W, Meyhack B, Rudolph H, Schweingruber A M, Hinnen A. Structural analysis of the two tandemly repeated acid phosphatase genes in yeast. Nucleic Acids Res. 1984;12:7722–7739. doi: 10.1093/nar/12.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergkamp R J M, Kool I M, Geerse R H, Planta R J. Multiple-copy integration of the α-galactosidase gene from Cyamopsis tetragonoloba into the ribosomal DNA of Kluyveromyces lactis. Curr Genet. 1992;21:365–370. doi: 10.1007/BF00351696. [DOI] [PubMed] [Google Scholar]
- 4.Bergman L W, McClinton D C, Madden S L, Preiss L H. Molecular analysis of the DNA sequences involved in the transcriptional regulation of the phosphate-repressible acid phosphatase gene (PHO5) of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1986;83:6070–6074. doi: 10.1073/pnas.83.16.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggin M D, Gibson T J, Hong G F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence termination. Proc Natl Acad Sci USA. 1983;80:3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondeau K, Boutur O, Boze H, Jung G, Moulin G, Galzy P. Development of high-cell-density fermentation for heterologous interleukin 1β production in Kluyveromyces lactis controlled by the PHO5 promoter. Appl Microbiol Biotechnol. 1994;41:324–329. doi: 10.1007/BF00221227. [DOI] [PubMed] [Google Scholar]
- 7.Blondeau K, Boze H, Jung G, Moulin G, Galzy P. Physiological approach to heterologous human serum albumin production by Kluyveromyces lactis in chemostat culture. Yeast. 1994;10:1297–1303. doi: 10.1002/yea.320101006. [DOI] [PubMed] [Google Scholar]
- 8.Boyer H, Roulland-Dussoix D. A complementary analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 9.Buckholz R G, Gleeson M A G. Yeast systems for the commercial production of heterologous proteins. Bio/Technology. 1991;9:1067–1072. doi: 10.1038/nbt1191-1067. [DOI] [PubMed] [Google Scholar]
- 10.Casadaban M J, Martínez-Arias A, Shapira S K, Chou J. β-Galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeasts. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- 11.Erickson P F, Minier L N, Lasher R S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoradiographic detection of antigens. J Immunol Methods. 1982;51:241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- 11a.Falcone, C. Personal communication.
- 12.Fermiñán E, Domínguez A. The KIPHO5 gene encoding a repressible acid phosphatase in the yeast Kluyveromyces lactis: cloning, sequencing and transcriptional analysis of the gene, and purification and properties of the enzymes. Microbiology. 1997;143:2615–2625. doi: 10.1099/00221287-143-8-2615. [DOI] [PubMed] [Google Scholar]
- 13.Fleer R, Chen X J, Amellal N, Yeh P, Fournier A, Guinet F, Gault N, Faucher D, Folliard F, Fukuhara H, Mayaux J F. High-level secretion of correctly processed recombinant human interleukin-1β in Kluyveromyces lactis. Gene. 1991;107:285–295. doi: 10.1016/0378-1119(91)90329-a. [DOI] [PubMed] [Google Scholar]
- 14.Fleer R, Yeh P, Maury I, Amellal N, Fournier A, Bacchetta F, Baduel P, Jung G, l’Hôte H, Becquart J, Fukuhara H, Mayaux J F. Stable multicopy vectors for high-level secretion of recombinant human serumalbumin in Kluyveromyces yeasts. Bio/Technology. 1991;9:968–975. doi: 10.1038/nbt1091-968. [DOI] [PubMed] [Google Scholar]
- 15.Fleer R. Engineering yeast for high level expression. Curr Opin Biotechnol. 1992;3:486–496. doi: 10.1016/0958-1669(92)90076-u. [DOI] [PubMed] [Google Scholar]
- 16.Haas J, Redl B, Friedlin E, Stoffler G. Isolation and analysis of the Penicillium chrysogenum phoA gene encoding a secreted phosphate-repressible acid phosphatase. Gene. 1992;113:129–133. doi: 10.1016/0378-1119(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 17.Haguenauer-Tsapis R, Hinnen A. A deletion that includes the signal peptidase cleavage site impairs processing, glycosylation and secretion of cell surface yeast acid phosphatase. Mol Cell Biol. 1984;4:2668–2675. doi: 10.1128/mcb.4.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 19.Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- 20.Hinnen A, Meyhack B, Heim J. Heterologous gene expression in yeast. In: Barr P J, Brake A J, Valenzuela P, editors. Yeast genetic engineering. London, England: Butterworths; 1989. pp. 193–213. [PubMed] [Google Scholar]
- 21.Hirst K, Fisher F, McAndrew P C, Goding C R. The transcription factor, the Cdk, its cyclin and their regulator: directing the transcriptional response to a nutritional signal. EMBO J. 1994;13:5410–5420. doi: 10.1002/j.1460-2075.1994.tb06876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadowaki H, Kadowaki T, Wondisford F E, Taylor S I. Use of polymerase chain reaction catalysed by Taq DNA polymerase for site-directed mutagenesis. Gene. 1989;76:161–166. doi: 10.1016/0378-1119(89)90018-8. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Martegani E, Forlani N, Mauri I, Porro D, Schleuning W D, Albergina L. Expression of high levels of human tissue plasminogen activator in yeast under the control of an inducible GAL promoter. Appl Microbiol Biotechnol. 1992;37:604–608. doi: 10.1007/BF00240734. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 325–355. [Google Scholar]
- 27.Morrissey J M. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakao J, Miyanohara A, Toh-e A, Matsubara K. Saccharomyces cerevisiae PHO5 promoter region: location and function of the upstream activation site. Mol Cell Biol. 1985;6:2613–2623. doi: 10.1128/mcb.6.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshima Y, Ogawa N, Harashima S. Regulation of phosphate synthesis in Saccharomyces cerevisiae: a review. Gene. 1996;179:171–177. doi: 10.1016/s0378-1119(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 30.Payne W E, Gannon P, Kaiser C A. An inducible acid phosphatase from the yeast Pichia pastoris: characterization of the gene and its product. Gene. 1995;163:19–26. doi: 10.1016/0378-1119(95)00379-k. [DOI] [PubMed] [Google Scholar]
- 31.Pesole G, Lotti M, Alberghina L, Saccone C. Evolutionary origin of non-universal CUGSer codon in some Candida species as inferred from a molecular phylogeny. Genetics. 1995;141:903–907. doi: 10.1093/genetics/141.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raeder U, Broda P. Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol. 1985;1:17–20. [Google Scholar]
- 33.Rentier-Deireu F, Swennen D, Mercier L, Lion M, Benrubi O, Martial J A. Molecular cloning and characterization of two forms of trout growth hormone cDNA: expression and secretion of tGH-II by Escherichia coli. DNA. 1989;8:109–117. doi: 10.1089/dna.1.1989.8.109. [DOI] [PubMed] [Google Scholar]
- 34.Romanos M A, Scorer C A, Clare J J. Foreign gene expression in yeasts: a review. Yeast. 1992;8:423–488. doi: 10.1002/yea.320080602. [DOI] [PubMed] [Google Scholar]
- 35.Rossolini F M, Riccio M L, Gallo E, Galeotti C L. Kluyveromyces lactis rDNA as a target for multiple integration by homologous recombination. Gene. 1992;119:75–81. doi: 10.1016/0378-1119(92)90068-z. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph H, Hinnen A. The yeast PHO5 promoter: phosphate-control elements and sequences mediating mRNA start-site selection. Proc Natl Acad Sci USA. 1987;84:1340–1344. doi: 10.1073/pnas.84.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J E, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sánchez M, Iglesias F J, Santamaría C, Domínguez A. Transformation of Kluyveromyces lactis by electroporation. Appl Environ Microbiol. 1993;59:2087–2092. doi: 10.1128/aem.59.7.2087-2092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swinkels B W, van Ooyen A J J, Bonekamp F J. The yeast Kluyveromyces lactis as an efficient host for heterologous gene expression. Antonie Leeuwenhoek. 1993;64:187–201. doi: 10.1007/BF00873027. [DOI] [PubMed] [Google Scholar]
- 40.Tokunaga M, Ishibashi M, Tatsuda D, Tokunaga H. Secretion of mouse α-amylase from Kluyveromyces lactis. Yeast. 1997;13:699–706. doi: 10.1002/(SICI)1097-0061(19970630)13:8<699::AID-YEA124>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berg J A, van der Laken K J, van Ooyen A J J, Renniers T C H M, Rietveld K, Schaap A, Brakes A J, Bishop R J, Shultz K, Moyer D, Richman M, Shuster J R. Kluyveromyces as a host for heterologous gene expression: expression and secretion of prochymosin. Bio/Technology. 1990;8:135–139. doi: 10.1038/nbt0290-135. [DOI] [PubMed] [Google Scholar]
- 43.Vogel K, Hörz W, Hinnen A. The two positively acting regulatory proteins PHO2 and PHO4 physically interact with PHO5 upstream activation regions. Mol Cell Biol. 1989;9:2050–2057. doi: 10.1128/mcb.9.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wesolowski-Louvel M, Breunig K D, Fukuhara H. Kluyveromyces lactis. In: Wolf K, editor. Nonconventional yeasts in biotechnology. Berlin, Germany: Springer-Verlag; 1996. pp. 139–201. [Google Scholar]
- 46.Wickerham L J. A critical evaluation of the nitrogen assimilation tests commonly used in the classification of yeast. J Bacteriol. 1946;52:293–301. doi: 10.1128/JB.52.3.293-301.1946. [DOI] [PubMed] [Google Scholar]