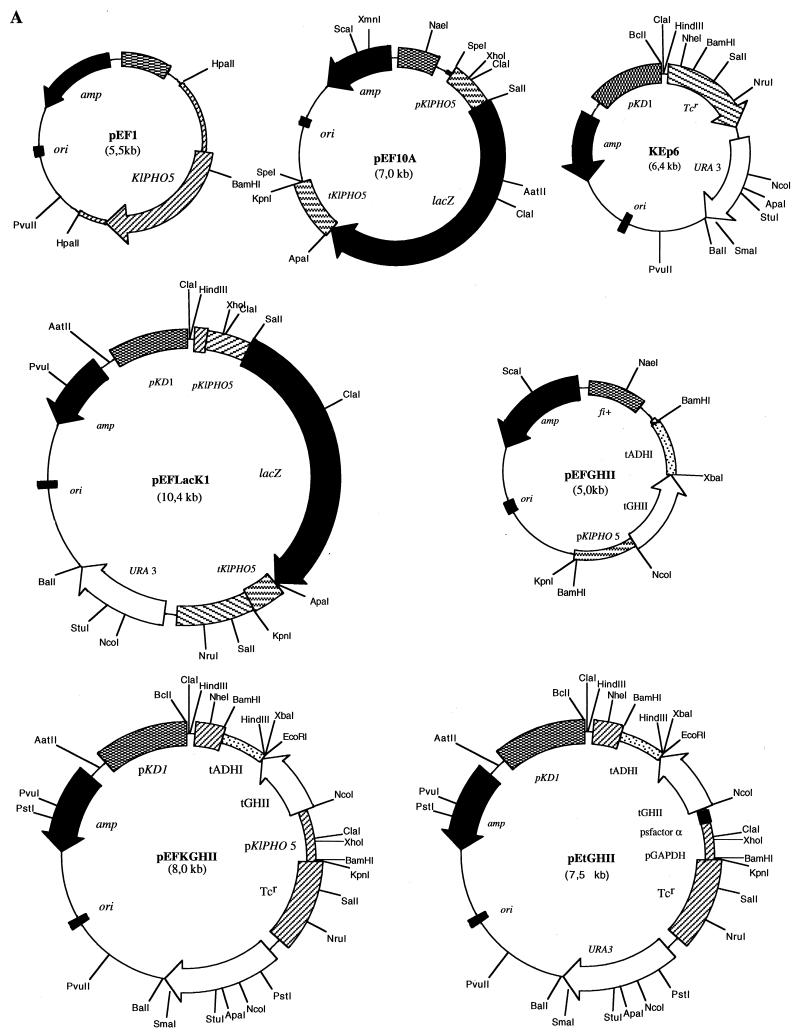
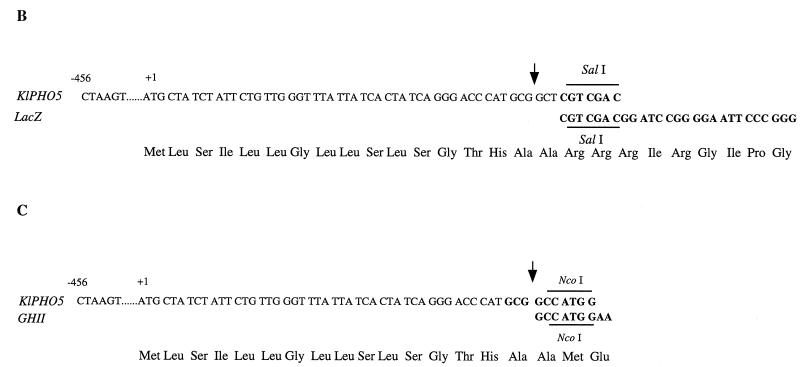
FIG. 1.
Construction of expression vectors. (A) The position of the signal peptide (SP) cleavage site in K. lactis APase encoded by KIPHO5 was predicted to be Ala16-Ala17 by the method of von Heijne (44) and confirmed by purification of mature APase and N-terminal sequencing (12). Plasmid pEF1, which contains the entire KIPHO5 gene of K. lactis, was used as template in PCR to isolate a 507-bp fragment comprising the KIPHO5 promoter and the first 17 codons of the KIPHO5 gene. After digestion with SalI this fragment was ligated to a 3,100-bp SalI-SalI fragment from pMC1871 (10) (Fig. 1B) containing the β-galactosidase gene. The KIPHO5 transcriptional termination signals (a 630-bp fragment [12]) were added 3′ to the SalI place of the KIPHO5 promoter β-galactosidase fusion, giving rise to plasmid pEF10A. (B and C) The whole 4,237-bp SpeI-SpeI fragment was inserted into the NheI site of the episomal plasmid KEp6 (45), yielding pEFLacK1. pEFGHII resulted from inserting a 504-bp fragment comprising the KIPHO5 promoter and the first 16 codons of the KIPHO5 gene with the NcoI-BamHI fragment containing the tGHII gene and the ADHI terminator (see Fig. 1A, plasmid pEtGHII). The whole 1,686-bp BamHI-BamHI fragment was inserted in the BamHI site of KEp6, giving rise to pEFKGHII (Fig. 1A).